Abstract
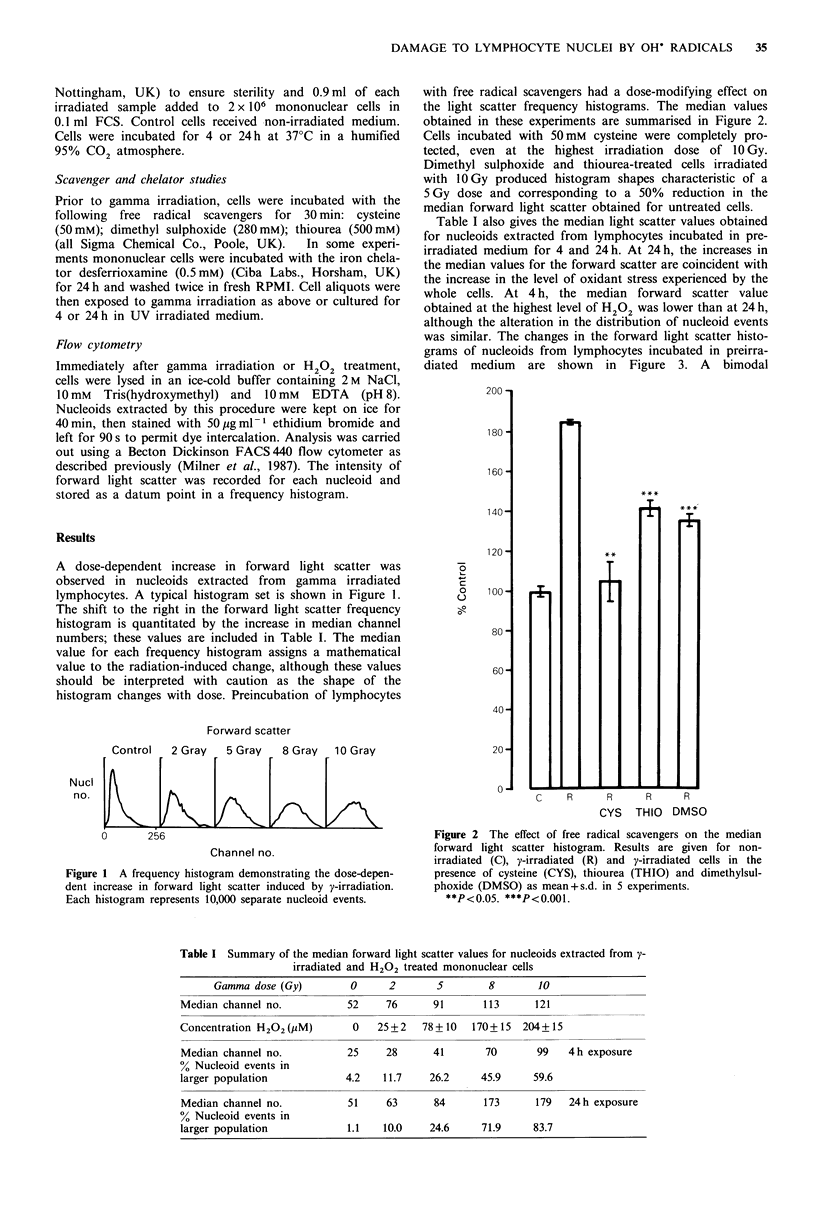
Normal human lymphocytes were exposed to OH. radicals produced indirectly by exposure to H2O2 or directly by gamma irradiation. Using a flow cytometry technique to measure changes in nucleoid size, it was found that generation of OH. in each system produced a characteristic relaxation in nuclear supercoiling. Exposure of cells to H2O2 produced a metal-dependent step-wise relaxation in extracted nucleoids, while gamma irradiation induced a gradual dose-dependent increase in nucleoid size. The site-specific metal-dependent changes produced in lymphocytes incubated in H2O2 should also occur in gamma irradiated cells, but the characteristic effects on nuclear supercoiling would not be detected within the background of random DNA damage. The importance of metals in maintaining the supercoiled loop configuration of DNA within the protein matrix suggests that free radical damage at metal locations may be particularly toxic for the cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan I. M., Lunec J., Salmon M., Bacon P. A. Reactive oxygen species selectively deplete normal T lymphocytes via a hydroxyl radical dependent mechanism. Scand J Immunol. 1987 Jul;26(1):47–53. doi: 10.1111/j.1365-3083.1987.tb02233.x. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A. Detection and repair of single-strand breaks in nuclear DNA. Nature. 1976 Oct 21;263(5579):679–682. doi: 10.1038/263679a0. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Brazell I. A., Jost E. Characterization of nuclear structures containing superhelical DNA. J Cell Sci. 1976 Nov;22(2):303–324. doi: 10.1242/jcs.22.2.303. [DOI] [PubMed] [Google Scholar]
- Cramp W. A., Lunec J., George A., Cresswell S., Lewis P. D., Chamberlain S., Harris G., Olsen I. The extent of bonding of newly synthesized DNA to parent template in unirradiated cells as a prediction of radiation sensitivity. Int J Radiat Biol Relat Stud Phys Chem Med. 1982 Feb;41(2):193–196. doi: 10.1080/09553008214550191. [DOI] [PubMed] [Google Scholar]
- Dijkwel P. A., Wenink P. W. Structural integrity of the nuclear matrix: differential effects of thiol agents and metal chelators. J Cell Sci. 1986 Aug;84:53–67. doi: 10.1242/jcs.84.1.53. [DOI] [PubMed] [Google Scholar]
- Elkind M. M. DNA damage and cell killing. Cause and effect? Cancer. 1985 Nov 15;56(10):2351–2363. doi: 10.1002/1097-0142(19851115)56:10<2351::aid-cncr2820561002>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Czapski G. The role and mechanism of metal ions and their complexes in enhancing damage in biological systems or in protecting these systems from the toxicity of O2-. J Free Radic Biol Med. 1986;2(1):3–11. doi: 10.1016/0748-5514(86)90117-0. [DOI] [PubMed] [Google Scholar]
- Hutchinson F. Chemical changes induced in DNA by ionizing radiation. Prog Nucleic Acid Res Mol Biol. 1985;32:115–154. doi: 10.1016/s0079-6603(08)60347-5. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Laemmli U. K. Evidence for two levels of DNA folding in histone-depleted HeLa interphase nuclei. J Mol Biol. 1982 Apr 5;156(2):309–324. doi: 10.1016/0022-2836(82)90331-x. [DOI] [PubMed] [Google Scholar]
- Lewis C. D., Laemmli U. K. Higher order metaphase chromosome structure: evidence for metalloprotein interactions. Cell. 1982 May;29(1):171–181. doi: 10.1016/0092-8674(82)90101-5. [DOI] [PubMed] [Google Scholar]
- Mello Filho A. C., Meneghini R. In vivo formation of single-strand breaks in DNA by hydrogen peroxide is mediated by the Haber-Weiss reaction. Biochim Biophys Acta. 1984 Feb 24;781(1-2):56–63. doi: 10.1016/0167-4781(84)90123-4. [DOI] [PubMed] [Google Scholar]
- Milner A. E., Vaughan A. T., Clark I. P. Measurement of DNA damage in mammalian cells using flow cytometry. Radiat Res. 1987 Apr;110(1):108–117. [PubMed] [Google Scholar]
- Rowley D. A., Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide and iron salts by superoxide- and ascorbate-dependent mechanisms: relevance to the pathology of rheumatoid disease. Clin Sci (Lond) 1983 Jun;64(6):649–653. doi: 10.1042/cs0640649. [DOI] [PubMed] [Google Scholar]
- Sagone A. L., Jr, Husney R., Guter H., Clark L. Effect of catalase on the proliferation of human lymphocytes to phorbol myristate acetate. J Immunol. 1984 Sep;133(3):1488–1494. [PubMed] [Google Scholar]
- Sasaki M. S., Matsubara S. Free radical scavenging in protection of human lymphocytes against chromosome aberration formation by gamma-ray irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1977 Nov;32(5):439–445. doi: 10.1080/09553007714551191. [DOI] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hyslop P. A., Hinshaw D. B., Spragg R. G., Sklar L. A., Cochrane C. G. Hydrogen peroxide-induced injury of cells and its prevention by inhibitors of poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4908–4912. doi: 10.1073/pnas.83.13.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstätter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Glutathione cycle activity and pyridine nucleotide levels in oxidant-induced injury of cells. J Clin Invest. 1985 Sep;76(3):1131–1139. doi: 10.1172/JCI112068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Pardoll D. M., Coffey D. S. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980 Nov;22(1 Pt 1):79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Ward J. F. Biochemistry of DNA lesions. Radiat Res Suppl. 1985;8:S103–S111. [PubMed] [Google Scholar]
- Ward J. F., Blakely W. F., Joner E. I. Mammalian cells are not killed by DNA single-strand breaks caused by hydroxyl radicals from hydrogen peroxide. Radiat Res. 1985 Sep;103(3):383–392. [PubMed] [Google Scholar]
- Ward J. F., Evans J. W., Limoli C. L., Calabro-Jones P. M. Radiation and hydrogen peroxide induced free radical damage to DNA. Br J Cancer Suppl. 1987 Jun;8:105–112. [PMC free article] [PubMed] [Google Scholar]
- Winterbourn C. C. Comparison of superoxide with other reducing agents in the biological production of hydroxyl radicals. Biochem J. 1979 Aug 15;182(2):625–628. doi: 10.1042/bj1820625. [DOI] [PMC free article] [PubMed] [Google Scholar]


