Abstract
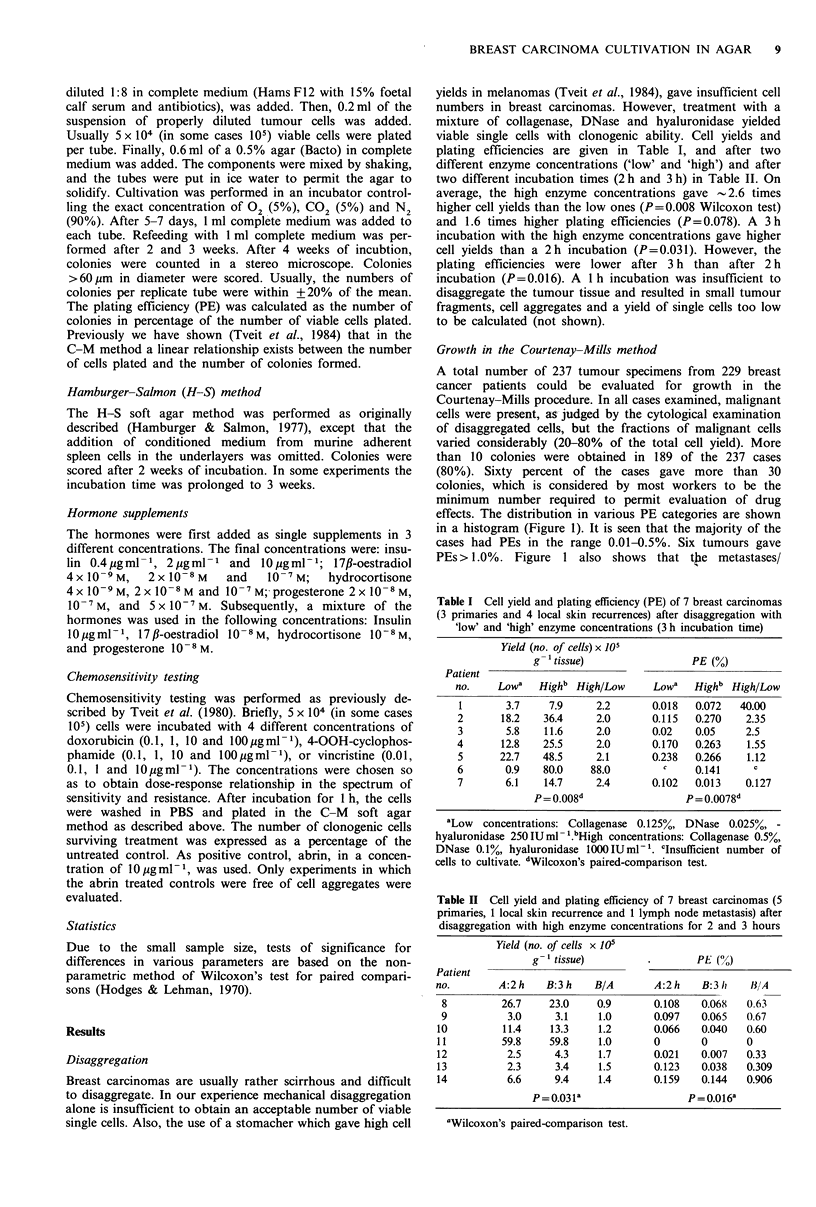
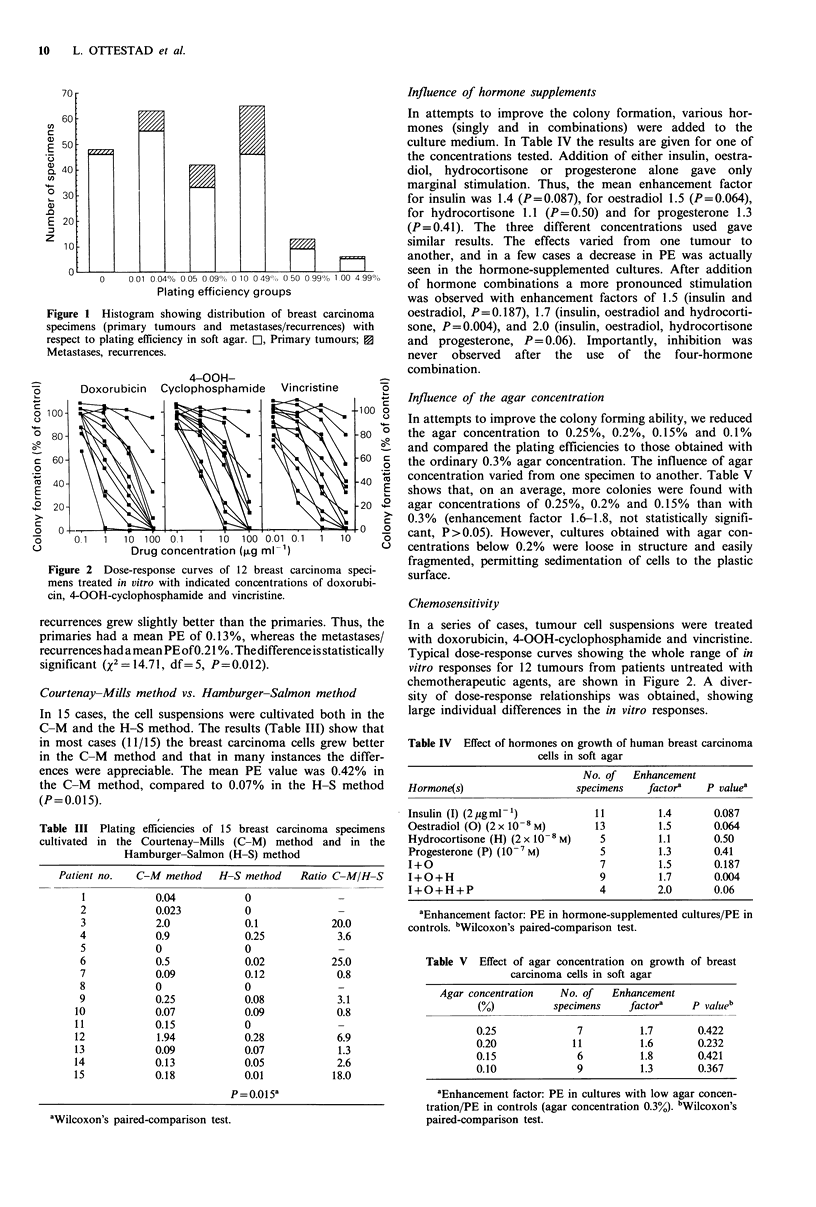
A total of 237 breast carcinomas have been studied with the Courtenay-Mills (C-M) soft agar method. Cell yields and plating efficiencies (PE) were recorded after various enzyme treatments. The highest cell yields and PEs were obtained with the combination of collagenase 0.5%, hyaluronidase 1000 IE ml-1 and DNase 0.1% and an incubation time of 2 h. Eighty percent of the specimens gave greater than 10 colonies, and 60% formed greater than 30 colonies permitting chemosensitivity studies. The C-M method gave significantly higher PEs than the Hamburger-Salmon (H-S) method. Hormone supplements (insulin, oestradiol, progesterone, hydrocortisone) and also reduced agar concentrations (less than 0.3%) gave marginal stimulation of colony formation. In chemosensitivity studies involving doxorubicin, vincristine and 4-OOH-cyclophosphamide, the C-M method gave dose-response relationships without plateaus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besch G. J., Tanner M. A., Howard S. P., Wolberg W. H., Gould M. N. Systematic optimization of the clonal growth of human primary breast carcinoma cells. Cancer Res. 1986 May;46(5):2306–2313. [PubMed] [Google Scholar]
- Besch G. J., Wolberg W. H., Gilchrist K. W., Voelkel J. G., Gould M. N. A comparison of methods for the production of monodispersed cell suspensions from human primary breast carcinomas. Breast Cancer Res Treat. 1983;3(1):15–22. doi: 10.1007/BF01806230. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D., Mills J. An in vitro colony assay for human tumours grown in immune-suppressed mice and treated in vivo with cytotoxic agents. Br J Cancer. 1978 Feb;37(2):261–268. doi: 10.1038/bjc.1978.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Hamburger A. W., White C. P., Dunn F. E., Citron M. L., Hummel S. Modulation of human tumor colony growth in soft agar by serum. Int J Cell Cloning. 1983 Sep;1(4):216–229. doi: 10.1002/stem.5530010403. [DOI] [PubMed] [Google Scholar]
- Hug V., Haynes M., Rashid R., Spitzer G., Blumenschen G., Hortobagyi G. Improved culture conditions for clonogenic growth of primary human breast tumours. Br J Cancer. 1984 Aug;50(2):207–213. doi: 10.1038/bjc.1984.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. E., Dean J. C., Young L. A., Salmon S. E. The human tumor clonogenic assay in human breast cancer. J Clin Oncol. 1985 Jan;3(1):92–97. doi: 10.1200/JCO.1985.3.1.92. [DOI] [PubMed] [Google Scholar]
- Rozencweig M., Hofmann V., Sanders C., Rombaut W., Früh U., Martz G. In vitro growth of human malignancies in a cloning assay. Recent Results Cancer Res. 1984;94:1–7. doi: 10.1007/978-3-642-82295-7_1. [DOI] [PubMed] [Google Scholar]
- Sandbach J., Von Hoff D. D., Clark G., Cruz A. B., Jr, Obrien M. Direct cloning of human breast cancer in soft agar culture. Cancer. 1982 Oct 1;50(7):1315–1321. doi: 10.1002/1097-0142(19821001)50:7<1315::aid-cncr2820500717>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Singletary S. E., Umbach G. E., Spitzer G., Drewinko B., Tomasovic B., Ajani J., Hug V., Blumenschein G. The human tumor stem cell assay revisited. Int J Cell Cloning. 1985 Mar;3(2):116–128. doi: 10.1002/stem.5530030205. [DOI] [PubMed] [Google Scholar]
- Slocum H. K., Pavelic Z. P., Kanter P. M., Nowak N. J., Rustum Y. M. The soft agar clonogenicity and characterization of cells obtained from human solid tumors by mechanical and enzymatic means. Cancer Chemother Pharmacol. 1981;6(3):219–225. doi: 10.1007/BF00256974. [DOI] [PubMed] [Google Scholar]
- Tveit K. M., Endresen L., Rugstad H. E., Fodstad O., Pihl A. Comparison of two soft-agar methods for assaying chemosensitivity of human tumours in vitro: malignant melanomas. Br J Cancer. 1981 Oct;44(4):539–544. doi: 10.1038/bjc.1981.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Olsnes S., Pihl A. In vitro sensitivity of human melanoma xenografts to cytotoxic drugs. Correlation with in vivo chemosensitivity. Int J Cancer. 1980 Dec 15;26(6):717–722. doi: 10.1002/ijc.2910260604. [DOI] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Pihl A. Cultivation of human melanomas in soft agar. Factors influencing plating efficiency and chemosensitivity. Int J Cancer. 1981 Sep 15;28(3):329–334. doi: 10.1002/ijc.2910280312. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D., Cowan J., Harris G., Reisdorf G. Human tumor cloning: feasibility and clinical correlations. Cancer Chemother Pharmacol. 1981;6(3):265–271. doi: 10.1007/BF00256979. [DOI] [PubMed] [Google Scholar]
- Whelan R. D., Hill B. T. The influence of agarose concentration on the cloning efficiency of a series of established human cell lines. Cell Biol Int Rep. 1981 Dec;5(12):1137–1142. doi: 10.1016/s0309-1651(81)80023-9. [DOI] [PubMed] [Google Scholar]



