Abstract
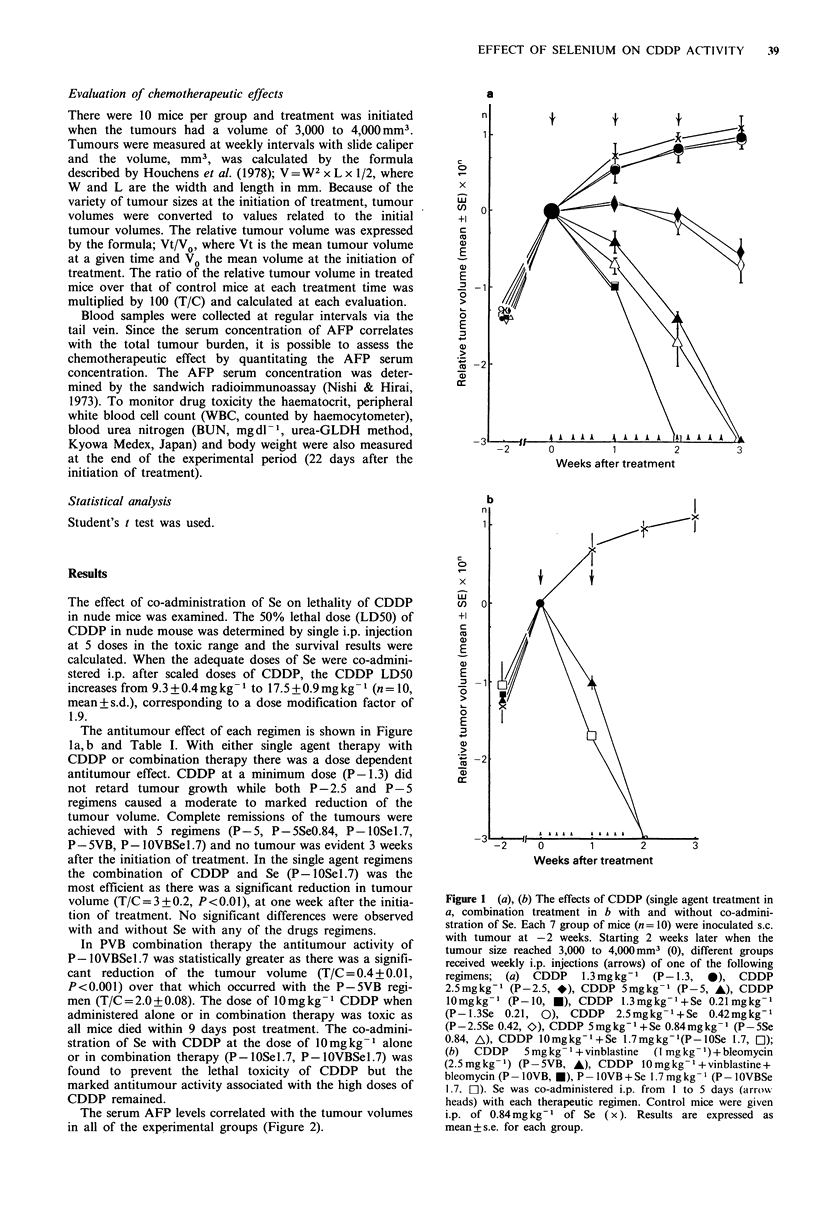
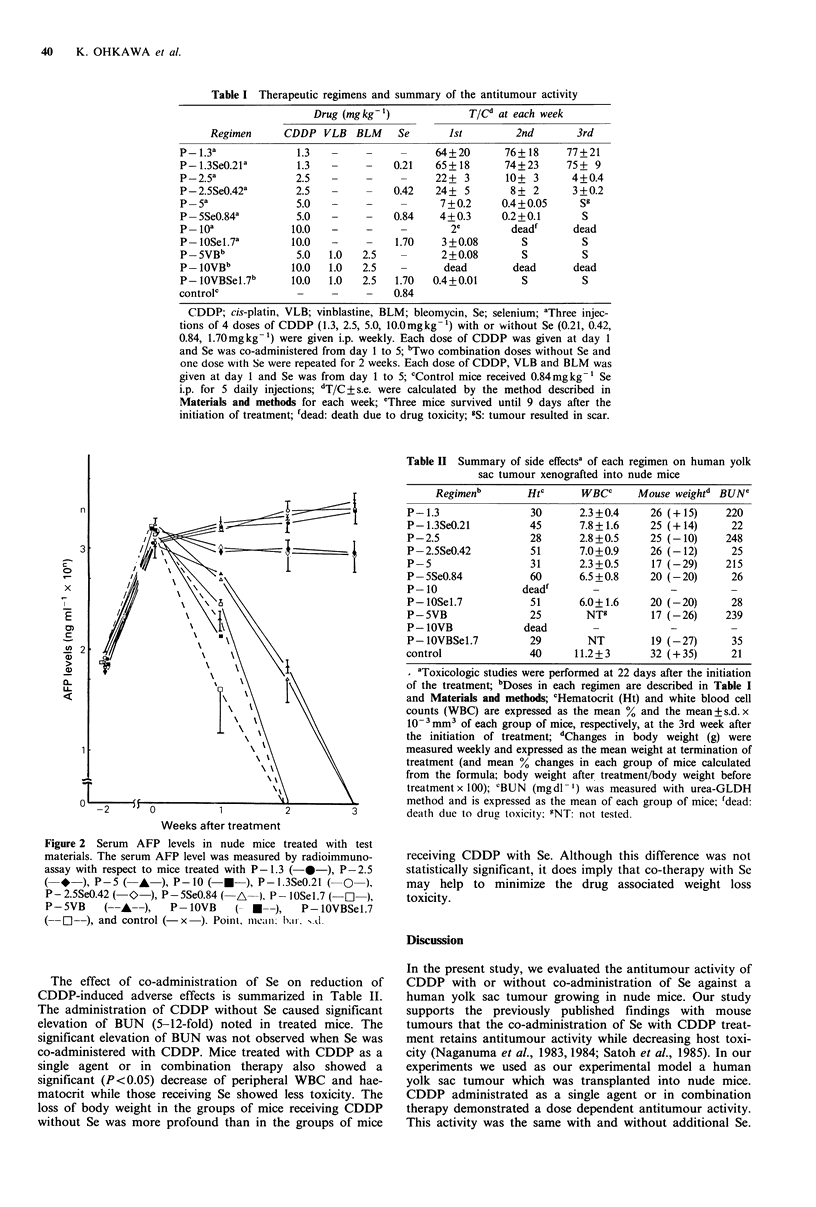
The therapeutic antitumour activity and host toxicity of cis-platin (CDDP), which was administered with selenium (sodium selenite) was studied on the growth of a human yolk sac tumour grown in nude mice. Treatment consisted of CDDP single agent chemotherapy (3 weeks) or preliminary PVB combination chemotherapy (CDDP + vinblastine + bleomycin, 2 weeks). Selenium was co-administered from day 1 to 5 with each therapeutic regimen. The administration of CDDP alone caused significant reduction in tumour burden but at higher doses there was significant host toxicity. The co-administration of selenium together with CDDP (CDDP: selenium, molar ratio = 3.5:1) did not affect the anti-tumour activity of CDDP but it did cause a decrease of parameters of host toxicity including lethality, increasing the 50% lethal dose (LD50) from 9.3 mg kg-1 to 17.5 mg kg-1. The parameters of host toxicity which were altered by selenium co-administration were nephrotoxicity, myeloid suppression and weight loss. Our study suggested that selenium co-administration allows higher doses of CDDP with reduction of apparent toxicity, resulting in a higher therapeutic index and possibly indicating a potential increase in the utilization of CDDP in clinical cancer chemotherapy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodenner D. L., Dedon P. C., Keng P. C., Katz J. C., Borch R. F. Selective protection against cis-diamminedichloroplatinum(II)-induced toxicity in kidney, gut, and bone marrow by diethyldithiocarbamate. Cancer Res. 1986 Jun;46(6):2751–2755. [PubMed] [Google Scholar]
- Borch R. F., Pleasants M. E. Inhibition of cis-platinum nephrotoxicity by diethyldithiocarbamate rescue in a rat model. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6611–6614. doi: 10.1073/pnas.76.12.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn L. H., Donohue J. P. Improved chemotherapy in disseminated testicular cancer. J Urol. 1977 Jan;117(1):65–69. doi: 10.1016/s0022-5347(17)58338-x. [DOI] [PubMed] [Google Scholar]
- Ganther H. E., Goudie C., Sunde M. L., Kopecky M. J., Wagner P. Selenium: relation to decreased toxicity of methylmercury added to diets containing tuna. Science. 1972 Mar 10;175(4026):1122–1124. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- Garnett M. C., Embleton M. J., Jacobs E., Baldwin R. W. Preparation and properties of a drug-carrier-antibody conjugate showing selective antibody-directed cytotoxicity in vitro. Int J Cancer. 1983 May 15;31(5):661–670. doi: 10.1002/ijc.2910310520. [DOI] [PubMed] [Google Scholar]
- Jacobs A. J., Harris M., Deppe G., DasGupta I., Cohen C. J. Treatment of recurrent and persistent germ cell tumors with cisplatin, vinblastine, and bleomycin. Obstet Gynecol. 1982 Jan;59(1):129–132. [PubMed] [Google Scholar]
- Kurman R. J., Norris H. J. Endodermal sinus tumor of the ovary: a clinical and pathologic analysis of 71 cases. Cancer. 1976 Dec;38(6):2404–2419. doi: 10.1002/1097-0142(197612)38:6<2404::aid-cncr2820380629>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Merrin C. E. Treatment of genitourinary tumours with cis-dichlorodiammineplatinum(II): experience in 250 patients. Cancer Treat Rep. 1979 Sep-Oct;63(9-10):1579–1584. [PubMed] [Google Scholar]
- Naganuma A., Satoh M., Imura N. Effect of selenite on renal toxicity and antitumor activity of cis-diamminedichloroplatinum in mice inoculated with Ehrlich ascites tumor cell. J Pharmacobiodyn. 1984 Mar;7(3):217–220. doi: 10.1248/bpb1978.7.217. [DOI] [PubMed] [Google Scholar]
- Naganuma A., Satoh M., Imura N. Prevention of lethal and renal toxicity of cis-diamminedichloroplatinum(II) by induction of metallothionein synthesis without compromising its antitumor activity in mice. Cancer Res. 1987 Feb 15;47(4):983–987. [PubMed] [Google Scholar]
- Naganuma A., Satoh M., Yokoyama M., Imura N. Selenium efficiently depressed toxic side effect of cis-diamminedichloroplatinum. Res Commun Chem Pathol Pharmacol. 1983 Oct;42(1):127–134. [PubMed] [Google Scholar]
- Ohkawa K., Hibi N., Tsukada Y. Evaluation of a conjugate of purified antibodies against human AFP-dextran-daunorubicin to human AFP-producing yolk sac tumor cell lines. Cancer Immunol Immunother. 1986;22(2):81–86. doi: 10.1007/BF00199119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa K., Tsukada Y., Hibi N., Umemoto N., Hara T. Selective in vitro and in vivo growth inhibition against human yolk sac tumor cell lines by purified antibody against human alpha-fetoprotein conjugated with mitomycin C via human serum albumin. Cancer Immunol Immunother. 1986;23(2):81–86. doi: 10.1007/BF00199811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols R. F., Corden B. J., Jacob J., Wesley M. N., Ostchega Y., Young R. C. High-dose cisplatin in hypertonic saline. Ann Intern Med. 1984 Jan;100(1):19–24. doi: 10.7326/0003-4819-100-1-19. [DOI] [PubMed] [Google Scholar]
- Prestayko A. W., D'Aoust J. C., Issell B. F., Crooke S. T. Cisplatin (cis-diamminedichloroplatinum II). Cancer Treat Rev. 1979 Mar;6(1):17–39. doi: 10.1016/s0305-7372(79)80057-2. [DOI] [PubMed] [Google Scholar]
- Tsukada Y., Bischof W. K., Hibi N., Hirai H., Hurwitz E., Sela M. Effect of a conjugate of daunomycin and antibodies to rat alpha-fetoprotein on the growth of alpha-fetoprotein-producing tumor cells. Proc Natl Acad Sci U S A. 1982 Jan;79(2):621–625. doi: 10.1073/pnas.79.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada Y., Hurwitz E., Kashi R., Sela M., Hibi N., Hara A., Hirai H. Chemotherapy by intravenous administration of conjugates of daunomycin with monoclonal and conventional anti-rat alpha-fetoprotein antibodies. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7896–7899. doi: 10.1073/pnas.79.24.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada Y., Kato Y., Umemoto N., Takeda Y., Hara T., Hirai H. An anti-alpha-fetoprotein antibody-daunorubicin conjugate with a novel poly-L-glutamic acid derivative as intermediate drug carrier. J Natl Cancer Inst. 1984 Sep;73(3):721–729. [PubMed] [Google Scholar]
- Tsukada Y., Ohkawa K., Hibi N. Suppression of human alpha-foetoprotein-producing hepatocellular carcinoma growth in nude mice by an anti alpha-foetoprotein antibody-daunorubicin conjugate with a poly-L-glutamic acid derivative as intermediate drug carrier. Br J Cancer. 1985 Jul;52(1):111–116. doi: 10.1038/bjc.1985.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. D., Stablein D. M., Einhorn L. H., Muggia F. M., Weiss R. B., Donohue J. P., Paulson D. F., Brunner K. W., Jacobs E. M., Spaulding J. T. Immediate adjuvant chemotherapy versus observation with treatment at relapse in pathological stage II testicular cancer. N Engl J Med. 1987 Dec 3;317(23):1433–1438. doi: 10.1056/NEJM198712033172303. [DOI] [PubMed] [Google Scholar]


