Abstract
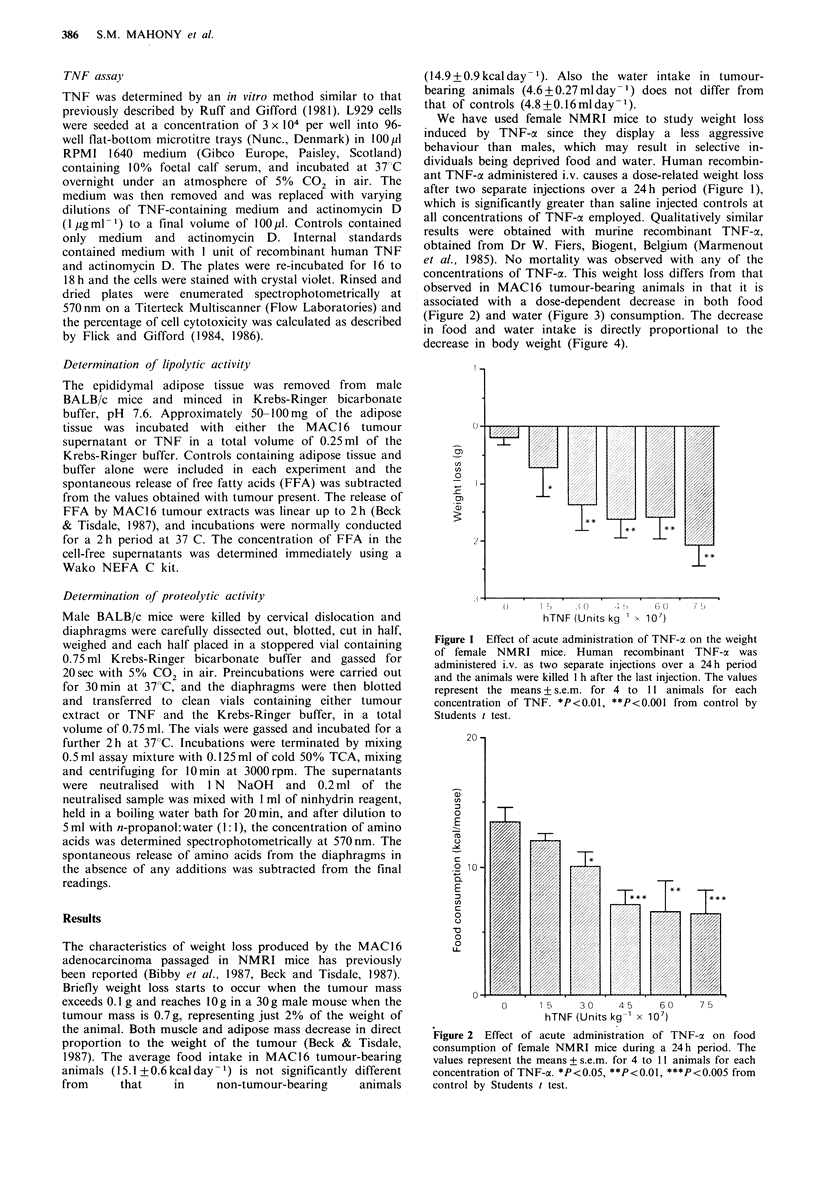
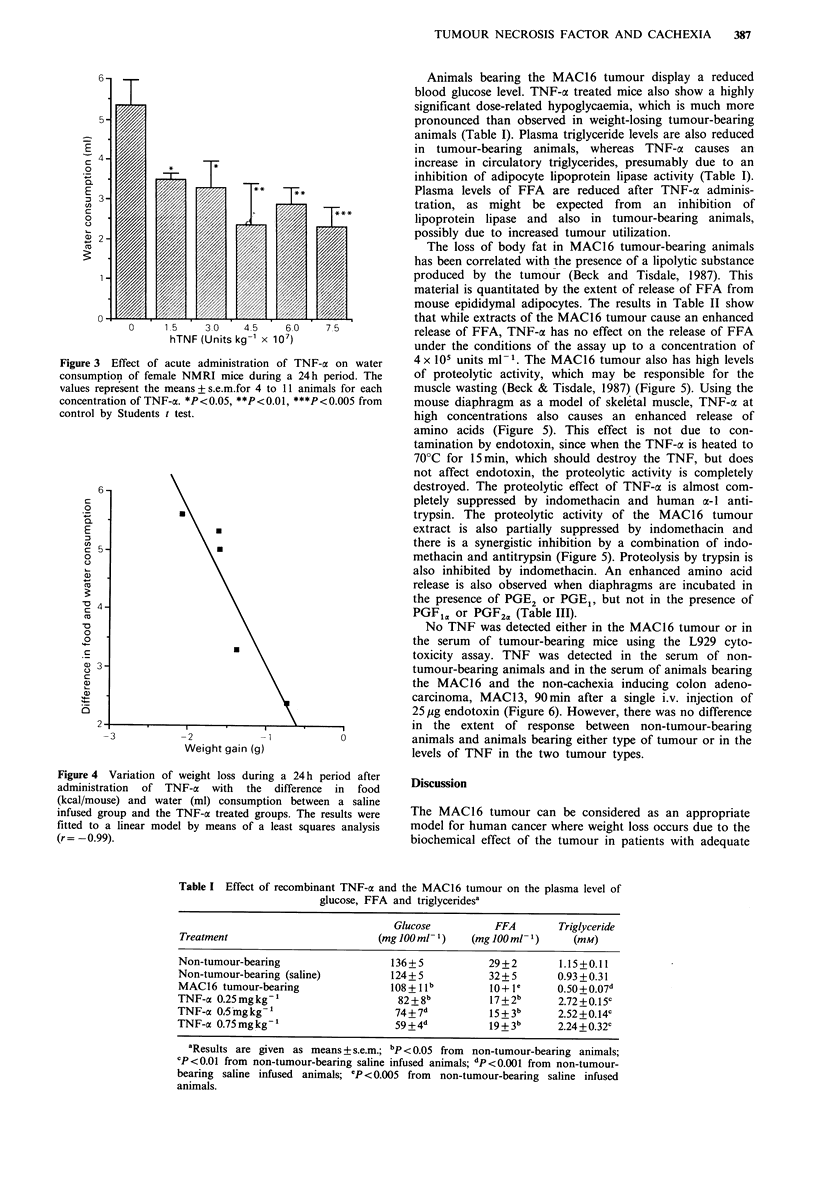
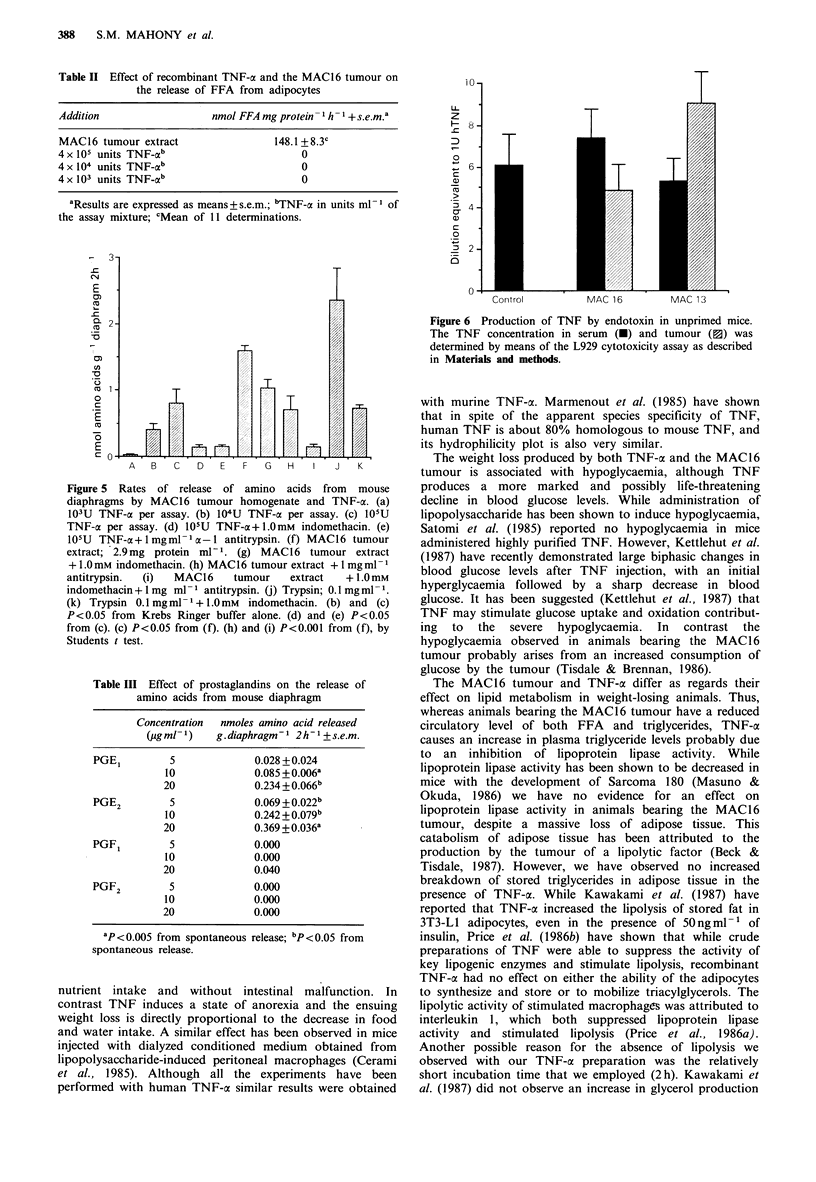
A comparison has been made of the cachectic effects produced by the transplantable murine adenocarcinoma of the mouse colon (MAC16) with tumour necrosis factor-alpha (cachectin). Tumour necrosis factor-alpha (TNF-alpha) produced a dose-related weight reduction that was accompanied by a decrease in both food and water intake. The degree of weight loss was directly proportional to the decreased food and water intake. In contrast weight loss produced by the MAC16 tumour occurred without a reduction in fluid or nutrient intake. Both the MAC16 tumour and TNF-alpha produced hypoglycaemia and a reduction in the circulatory level of free fatty acids (FFA), but had opposite effects on the level of plasma triglycerides with the MAC16 tumour-induced cachexia causing a decrease and TNF-alpha producing an increase. The MAC16 tumour elaborated a lipolytic factor which caused an immediate release of FFA from adipose tissue. In contrast TNF-alpha had no effect on mobilization of adipose triglycerides over a short time period. Both TNF-alpha and extracts from the MAC16 tumour caused an enhanced release of amino acids from mouse diaphragm, which was suppressible with indomethacin and heat labile. No TNF was detected in the MAC16 tumour or in the serum of tumour-bearing animals. Both tumour and non-tumour-bearing animals responded with a similar elevation of their serum TNF levels 90 min after a single injection of endotoxin. It is concluded that weight loss produced by TNF-alpha arises from an anorexic effect and that this differs from the complex metabolic changes associated with cancer cachexia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck S. A., Tisdale M. J. Production of lipolytic and proteolytic factors by a murine tumor-producing cachexia in the host. Cancer Res. 1987 Nov 15;47(22):5919–5923. [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Bibby M. C., Double J. A., Ali S. A., Fearon K. C., Brennan R. A., Tisdale M. J. Characterization of a transplantable adenocarcinoma of the mouse colon producing cachexia in recipient animals. J Natl Cancer Inst. 1987 Mar;78(3):539–546. [PubMed] [Google Scholar]
- Cerami A., Ikeda Y., Le Trang N., Hotez P. J., Beutler B. Weight loss associated with an endotoxin-induced mediator from peritoneal macrophages: the role of cachectin (tumor necrosis factor). Immunol Lett. 1985;11(3-4):173–177. doi: 10.1016/0165-2478(85)90165-8. [DOI] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984 Mar 30;68(1-2):167–175. doi: 10.1016/0022-1759(84)90147-9. [DOI] [PubMed] [Google Scholar]
- Flick D. A., Gifford G. E. Production of tumor necrosis factor in unprimed mice: mechanism of endotoxin-mediated tumor necrosis. Immunobiology. 1986 Jul;171(4-5):320–328. doi: 10.1016/S0171-2985(86)80064-X. [DOI] [PubMed] [Google Scholar]
- Kawakami M., Murase T., Ogawa H., Ishibashi S., Mori N., Takaku F., Shibata S. Human recombinant TNF suppresses lipoprotein lipase activity and stimulates lipolysis in 3T3-L1 cells. J Biochem. 1987 Feb;101(2):331–338. doi: 10.1093/oxfordjournals.jbchem.a121917. [DOI] [PubMed] [Google Scholar]
- Kettelhut I. C., Fiers W., Goldberg A. L. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4273–4277. doi: 10.1073/pnas.84.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmenout A., Fransen L., Tavernier J., Van der Heyden J., Tizard R., Kawashima E., Shaw A., Johnson M. J., Semon D., Müller R. Molecular cloning and expression of human tumor necrosis factor and comparison with mouse tumor necrosis factor. Eur J Biochem. 1985 Nov 4;152(3):515–522. doi: 10.1111/j.1432-1033.1985.tb09226.x. [DOI] [PubMed] [Google Scholar]
- Masuno H., Okuda H. Hepatic triacylglycerol lipase in circulating blood of normal and tumor-bearing mice and its hydrolysis of very-low-density lipoprotein and synthetic acylglycerols. Biochim Biophys Acta. 1986 Dec 5;879(3):339–344. [PubMed] [Google Scholar]
- Price S. R., Mizel S. B., Pekala P. H. Regulation of lipoprotein lipase synthesis and 3T3-L1 adipocyte metabolism by recombinant interleukin 1. Biochim Biophys Acta. 1986 Dec 19;889(3):374–381. doi: 10.1016/0167-4889(86)90201-6. [DOI] [PubMed] [Google Scholar]
- Price S. R., Olivecrona T., Pekala P. H. Regulation of lipoprotein lipase synthesis by recombinant tumor necrosis factor--the primary regulatory role of the hormone in 3T3-L1 adipocytes. Arch Biochem Biophys. 1986 Dec;251(2):738–746. doi: 10.1016/0003-9861(86)90384-x. [DOI] [PubMed] [Google Scholar]
- Rodemann H. P., Goldberg A. L. Arachidonic acid, prostaglandin E2 and F2 alpha influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem. 1982 Feb 25;257(4):1632–1638. [PubMed] [Google Scholar]
- Satomi N., Sakurai A., Haranaka K. Relationship of hypoglycemia to tumor necrosis factor production and antitumor activity: role of glucose, insulin, and macrophages. J Natl Cancer Inst. 1985 Jun;74(6):1255–1260. [PubMed] [Google Scholar]
- Tisdale M. J., Brennan R. A. Metabolic substrate utilization by a tumour cell line which induces cachexia in vivo. Br J Cancer. 1986 Oct;54(4):601–606. doi: 10.1038/bjc.1986.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]


