Abstract
The activation of T cells is tightly controlled by many positive and negative regulatory processes. This fine-tuning allows productive immunity to pathogens while minimizing the risk of autoimmunity. One negative regulatory mechanism is clonal anergy, which is a hyporesponsive state that occurs when T cells are activated through the T-cell antigen receptor in the absence of appropriate co-stimulatory signals. Recent studies have confirmed a crucial role for defective Ras activation in mediating this hyporesponsive state. Diminished Ras activation can, in part, be explained by the upregulated expression of diacylglycerol kinases (DGKs), which phosphorylate diacylglycerol and restrict Ras guanyl releasing protein 1 (RasGRP1)-dependent activation of Ras. Increased expression of DGKs is probably transcriptional and is accompanied by augmented expression of additional negative regulators, including the transcription factors early growth response (Egr) 2 and Egr3, and the E3 ubiquitin ligases known as gene related to anergy in lymphocytes (GRAIL) and Casitas B-cell lymphoma-b (Cbl-b). A model is emerging for how these factors are regulated to control T-cell responsiveness.
Keywords: T-cell activation, signal transduction, Ras, diacylglycerol kinase, anergy
T-cell activation and the function of CD28
T-cell activation is driven by the T-cell receptor (TCR), which recognizes peptide antigens bound to class I or class II major histocompatibility complex (MHC) molecules. Optimal T-cell activation occurs when T cells are stimulated by antigen-presenting cells (APCs) such as dendritic cells, which themselves have been activated to also express co-stimulatory molecules that act in concert with MHC/peptide–TCR interactions. The most crucial set of co-stimulatory ligands are CD80/B7-1 and CD86/B7-2, which engage the CD28 receptor expressed by T cells. CD28 co-stimulation has been reported to augment cytokine gene expression, promote cytokine messenger RNA stability, increase cellular proliferation, drive increased glucose uptake and utilization, and support cell survival (Boise et al, 1995; Frauwirth et al, 2002; Lindstein et al, 1989). In addition, ligation of CD28 maintains T-cell responsiveness on subsequent restimulation. TCR engagement in the absence of co-stimulation results in a hyporesponsive state termed anergy (Schwartz, 2003). T-cell anergy is thought to represent one mechanism of peripheral tolerance that maintains T cells in an ‘off' state when full immune activation is not desirable, such as in response to self-antigens. There has been substantial interest in gaining a greater understanding of the molecular mechanisms underlying anergy, as promoting this state could represent a strategy for the treatment of autoimmunity and allergic conditions, whereas preventing or reversing this state could have therapeutic value in the treatment of cancer and chronic infections.
Conditions that induce T-cell anergy
Anergy has been observed both in vitro and in vivo. In vitro anergy was first characterized by the Schwartz laboratory in the 1980s using CD4+ T helper 1 (Th1) cell clones (Jenkins & Schwartz, 1987). They found that when T cells encountered antigens presented by chemically fixed APCs—which presumably failed to upregulate co-stimulatory ligands—the T cells proliferated poorly and made less interleukin-2 (IL-2) than when live APCs were used. In addition, when the T cells were recovered and restimulated, they were found to be hyporesponsive. The induction of anergy has also been observed with other agents that engage the TCR complex alone, such as purified MHC–peptide complexes displayed on a planar lipid bilayer, anti-CD3 monoclonal antibody, the mitogen concanavalin A, or peptides presented by inefficient APCs such as small resting B cells (Gajewski et al, 1994a). In addition to a nearly complete block in IL-2 production and proliferation, anergic T cells show reduced secretion of other cytokines such as interferon-γ (IFN-γ) and IL-3. The anergic state can be long-lived and stable for weeks, but in these model systems it is reversible by the addition of exogenous cytokines such as IL-2 or IL-15 (Beverly et al, 1992; Essery et al, 1988). This reversibility has motivated further investigation of anergy in the settings of cancer and chronic infection, in which restoration of correct T-cell function is one of the main therapeutic goals.
An anergy-like state can also be induced in vitro by altered peptide ligands, which are peptides with minor sequence differences from native agonist antigenic peptides and that give lower avidity TCR ligation (Sloan-Lancaster et al, 1993). Engagement of the TCR with altered peptide ligands even in the presence of co-stimulation induces a state of hyporesponsiveness that resembles anergy. Recently, Korb and colleagues reported that low doses (fewer than 10 peptide/MHC complexes per APC) of agonist peptides can also induce an anergy-like T-cell dysfunctional state (Korb et al, 1999; Mirshahidi et al, 2001). It is not clear whether the molecular regulation of these types of T-cell dysfunction is the same as that which has been determined for classical anergy—that is, agonist TCR ligation in the absence of co-stimulation.
In addition to these experimental models of anergy induction in vitro, several models of anergy induction in vivo have been explored. Systemic delivery of superantigens, adoptive transfer of TCR transgenic T cells into hosts that express the cognate antigen as a self-antigen, and the administration of soluble peptide antigen into TCR transgenic mice have all been shown to result in an anergic state (Dubois et al, 1998; Kawabe & Ochi, 1990; Kearney et al, 1994; Pape et al, 1998). Superantigens such as staphylococcal enterotoxin B are proteins that bridge the lateral surface of MHC class II molecules and the TCR Vβ domain outside the antigen recognition clefts. This interaction activates a large portion of the T-cell population independent of TCR antigenic specificity. After the administration of superantigens, T cells initially expand then decrease in number, and the remaining T cells become hyporesponsive by a mechanism that resembles anergy (Kawabe & Ochi, 1990). Anergy can similarly be induced in TCR transgenic T cells, either by injection with the soluble antigen that is recognized by the T cells in the absence of adjuvant, or by adoptive transfer into hosts that constitutively express antigen on self-tissues—so-called adaptive tolerance (Kearney et al, 1994; Pape et al, 1998). It is not yet clear whether the fine details of the molecular alterations associated with T-cell dysfunction are identical in each of these model systems. Nonetheless, these observations argue that the method by which antigen is delivered for immunomodulatory purposes in vivo can have a profound impact on the functional outcome of antigen administration.
Signalling in the induction phase of anergy
On the basis of the in vitro anergy model systems, the signal transduction events that drive the induction of anergy rather than full T-cell activation seem to involve the disproportionate over-activation of calcium/nuclear factor of activated T cells (NFAT) signalling compared with other biochemical signalling intermediates, such as the Ras/mitogen-activated protein (MAP) kinase pathway. The addition of calcium ionophores alone—such as ionomycin—is sufficient to induce anergy of T cells. Conversely, induction of anergy with CD3 monoclonal antibodies can be prevented by cyclosporin A, which blocks NFAT-dependent signalling (Chai & Lechler, 1997). Rao and colleagues were the first to publish on the gene expression perturbations in T cells anergized with ionomycin, and they observed upregulated expression of numerous transcripts encoding putative negative regulatory functions during T-cell activation (Macian et al, 2002). The induction of these genes was dependent on NFAT, as the upregulation failed to occur in T cells from NFAT-deficient mice.
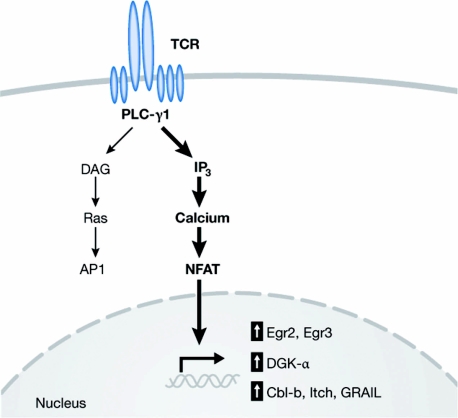
Several lines of evidence indicate that the induction of anergy involves increased expression of negative regulatory factors. First, the protein synthesis inhibitor cycloheximide prevents anergy induction, suggesting that the synthesis of a new protein (or proteins) is required to mediate blunted TCR-dependent T-cell activation. Second, transient heterokaryon fusion experiments between normal and anergic T cells have indicated that a large proportion of the anergic state can be accounted for by a dominant negative effect (Telander et al, 1999). Together, these results suggest a model in which excessive calcium/NFAT signalling results in transcriptional upregulation of negative regulatory proteins that inhibit correct TCR/CD28 signalling (Fig 1).
Figure 1.
Signals leading to the induction of anergy. Most data are consistent with a model in which excessive calcium/NFAT signalling leads to upregulated expression of negative regulatory factors that inhibit aspects of TCR/CD28-dependent signalling. Identified factors include the lipid kinase DGK-α; the E3 ubiquitin ligases Cbl-b, Itch and GRAIL; and the transcription factors Egr1 and Egr2. AP1, activator protein 1; Cbl-b, Casitas B-cell lymphoma-b; DAG, diacylglycerol; DGK-α, diacylglycerol kinase-α; Egr, early growth response; GRAIL, gene related to anergy in lymphocytes; IP3, inositol 1,4,5-trisphosphate; NFAT, nuclear factor of activated T cells; PLC-γ1, phospholipase C-γ1; TCR, T-cell receptor.
Defective Ras activation and the anergic state
Although anergic T cells show defective cytokine production in response to TCR/CD28 ligation, they still produce meaningful levels of cytokines in response to the pharmacological agents phorbol 12-myristate 13-acetate (PMA) plus ionomycin. This supports a proximal signal transduction defect in the anergic state. The analysis of many signalling pathways has consistently revealed defective activation of the MAP kinases ERK and JNK in response to TCR-based signalling in several model systems (Li et al, 1996). Moving upstream from MAP kinases, activation of Ras itself is disrupted in anergic T cells (Fields et al, 1996a). Consistent with diminished activation of Ras/MAP kinase signalling, activator protein 1 (AP1)-dependent transactivation, which lies downstream from Ras, has also been found to be defective (Fields et al, 1996b; Kang et al, 1992).
Despite convincing correlative data implicating blunted Ras pathway signalling in anergic T cells, mechanistic experiments proving causality was lacking. The desired experiment was to introduce active Ras into already anergized T cells and to determine whether MAP kinase signalling and cytokine production were restored. However, genetic manipulation of non-proliferative normal T-cell populations proved difficult using conventional strategies. This technical barrier was solved through the use of T cells from Coxsackie and adenovirus receptor (CAR) transgenic mice. T cells from these mice are efficiently transduced with adenoviral vectors without the need for cell proliferation (Wan et al, 2000). When anergic T cells were transduced with an adenovirus encoding constitutively active Ras, IL-2 production and MAP kinase activity were restored (Zha et al, 2006). These data strongly suggest that defective Ras signalling is the main functional defect in the anergic state, at least in the model systems of anergy studied so far. It should be noted that Mondino and colleagues performed a similar experiment transducing normal T cells with a retroviral vector encoding active Ras and did not observe resistance to anergy induction (Crespi et al, 2002). Although the reasons why different results were obtained in these various model systems are not clear, the requirement for T-cell activation, proliferation and retroviral integration in order to gain active Ras expression using the retroviral approach—in contrast to the ability to introduce active Ras directly into anergic T cells using the adenoviral system—is an obvious experimental difference.
The experiments described above suggested that the expression of a protein (or proteins) that has a negative impact on Ras activation is upregulated in anergic T cells. A previous hypothesis proposed that increased activation of an alternative GTPase, Rap1, suppresses Ras activation in anergic cells (Boussiotis et al, 1997). However, this model was not supported by studies of Rap1 transgenic mice or of mice deficient in the adaptor protein CrkL that contributes to Rap1 activation, as data from these experiments did not suggest an inhibitory role for this pathway in normal T cells (Peterson et al, 2003; Sebzda et al, 2002). Ras is activated by a family of guanine nucleotide exchange factors (GEFs) that catalyse GDP release from Ras, which enables subsequent GTP binding and the recruitment of the serine/threonine kinase Raf1. The main GEF described in most model systems is Sos (Son of sevenless). Sos is constitutively bound to the adaptor protein Grb2 (growth factor receptor-bound protein 2), which can be recruited through its SH2 domain to tyrosine phosphorylated proteins that are generated after receptor triggering. In T cells, Grb2–Sos complexes are thought to be recruited to the membrane-associated linker for activated T cells (LAT) following TCR ligation (Wange, 2000). However, recent evidence has suggested that the dominant Ras GEF involved in TCR-based signalling is the diacylglycerol (DAG)-binding molecule Ras guanyl releasing protein 1 (RasGRP1). RasGRP1-deficient mice show a block in thymic selection (Dower et al, 2000), indicating a non-redundant role for this GEF in T-cell development. The Weiss laboratory reported that in Jurkat T cells, TCR-mediated Ras activation was dependent on RasGRP1 and could not be compensated for by Grb2–Sos (Roose et al, 2007). Over-expression of RasGRP1 also renders Jurkat cells hypersensitive to TCR-stimulated ERK activation and IL-2 secretion (Ebinu et al, 2000). Therefore, if anergic T cells show defective Ras activation, this block might be expected to be at the level of RasGRP1. Consistent with this idea, anergic T cells can be rescued by the addition of exogenous PMA (Li et al, 1996), which theoretically could directly recruit RasGRP1 to membrane compartments and restore Ras activation.
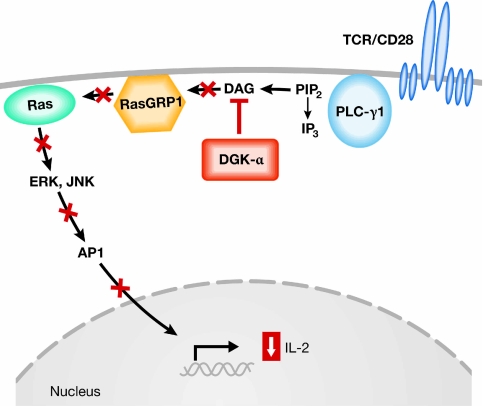
Gene expression profiling of anergic T cells compared with control T cells was performed to identify putative negative regulators of RasGRP1-dependent Ras activation that are upregulated in the anergic state. This analysis revealed a single main candidate, diacylglycerol kinase-α (DGK-α; Zha et al, 2006). Increased expression of DGK-α in anergic T cells was also observed by the Rao laboratory (Heissmeyer et al, 2004; Macian et al, 2002). DGKs phosphorylate DAG, thus converting it to phosphatidic acid and depleting available DAG that otherwise could activate RasGRP1 (Tognon et al, 1998). Phosphatidic acid also has signalling properties of its own that could contribute to altered T-cell signalling (Mor et al, 2007). The kinetics of DGK-α upregulation parallel the acquisition of T-cell dysfunction, both of which occur at around 16–24 h after the initiation of anergy induction. Zha and colleagues introduced an adenoviral vector encoding DGK-α into CAR transgenic T cells, and this inhibited MAP kinase signalling and IL-2 production. Conversely, inhibition of DGK activity using a pharmacologic inhibitor could restore a large fraction of IL-2 production by anergic T cells (Zha et al, 2006). Expression of DGK-α also resulted in diminished recruitment of RasGRP1 to the plasma membrane, supporting a link to RasGRP1-dependent Ras activation. Corroborative data have been obtained using T cells from mice made genetically deficient in DGK-α or the related family member DGK-ζ. T cells from either of these strains of mice were hyperresponsive in vitro and showed significant resistance to anergy induction through B7 blockade in vitro, or by using superantigen injection in vivo (Olenchock et al, 2006). Together, these results have identified DGK molecules as crucial negative regulators of TCR-mediated Ras activation in the anergic state. The fact that DGKs are lipid kinases that can be inhibited with chemical compounds opens the possibility of pharmacological inhibition of DGKs to augment T-cell function therapeutically in vivo. A model for the role of DGKs in suppressing Ras activation in the anergic state is depicted in Fig 2.
Figure 2.
Model for defective T-cell receptor signalling through upregulated expression of diacylglycerol kinases. Upregulated expression of DGK-α mediates phosphorylation of DAG, which leads to its decreased availability for activation of Ras through RasGRP1. This results in diminished activation of the downstream effectors ERK and JNK, with concomitant blunting of AP1-dependent transcription. AP1, activator protein 1; DAG, diacylglycerol; DGK-α, diacylglycerol kinase-α; IL-2, interleukin-2; IP3, inositol 1,4,5-trisphosphate; PIP2, phosphatidylinositol bisphosphate; PLC-γ1, phospholipase C-γ1; RasGRP1, Ras guanyl releasing protein 1; TCR, T-cell receptor.
Potential role for Egr family transcription factors
The increased expression of DGKs in T-cell anergy occurs at the mRNA level, and can be mimicked by ionomycin and blocked by cyclosporin A (Zha et al, 2006); therefore, it is probably regulated at the transcription level in an NFAT-dependent manner. However, preliminary analysis of the putative DGK-α promoter has not revealed any canonical NFAT sites (Y. Zheng and T.F.G., unpublished data); therefore, the NFAT-dependency of DGK-α expression might occur through unconventional NFAT sites, or be mediated through another intermediate transcription factor, which is itself NFAT-dependent. One attractive hypothesis is that this occurs through early growth response gene (Egr) 2 and Egr3, which are also upregulated in anergic T cells (Safford et al, 2005). Egr2 and Egr3 are transcription factors that bind to DNA through a conserved zinc-finger domain (Chavrier et al, 1988; Patwardhan et al, 1991), and we have observed two canonical Egr-binding sites in the putative DGK-α promoter (Y. Zheng and T.F.G., unpublished data). Overexpression of Egr2 and Egr3 reduced IL-2 production in T cells and has been shown to upregulate Casitas B-cell lymphoma-b (Cbl-b). Conversely, Egr3-deficient T cells were relatively resistant to anergy induction in an in vivo peptide-induced anergy model (Safford et al, 2005). As discussed below, Cbl-b has also been implicated as a contributor to the anergic state and is upregulated on Egr2/3 overexpression, arguing perhaps that Egr proteins are regulating the transcription of several anergy-associated genes.
E3 ubiquitin ligases and the anergic state
In addition to DGK-α, several other putative negative regulators of TCR-based signalling have been shown to be upregulated in anergic T cells. Perhaps the best characterized category of negative regulators are the E3 ubiquitin ligases. Gene expression profiling has revealed that at least three different E3 ubiquitin ligases—Cbl-b, gene related to anergy in lymphocytes (GRAIL) and Itch—are upregulated at the mRNA and protein levels during ionomycin-induced anergy (Heissmeyer et al, 2004). Mechanistic experiments have been performed examining the contribution of each of these factors to the anergic phenotype and peripheral tolerance.
Cbl-b is a RING-type E3 protein and Cbl-b knockout mice develop spontaneous, generalized autoimmunity. Although thymocyte development seems to be normal in Cbl-b knockout mice, peripheral T cells are hyperproliferative and produce more IL-2 on TCR activation compared with wild-type mice (Bachmaier et al, 2000). Loss of Cbl-b also results in impaired induction of T-cell tolerance both in vitro and in vivo (Jeon et al, 2004).
Itch is a HECT-type E3 protein and Itch knockout mice spontaneously develop a systemic inflammatory disease, indicating the failure of peripheral tolerance (Perry et al, 1998). Itch-deficient T cells show an activated phenotype and enhanced proliferation on TCR engagement. In addition, Itch overexpression in Jurkat cells reduced AP1-driven luciferase activity under both resting and anti-CD3 stimulated conditions (Fang et al, 2002).
GRAIL is a RING-type E3 protein. This gene was first identified by comparing changes in gene expression after TCR signalling in the presence or absence of B7 co-stimulation. The mRNA for GRAIL was found to be induced in anergic CD4 T cells (Anandasabapathy et al, 2003). Overexpression of GRAIL in naive CD4 T cells nearly abolished IL-2 production and proliferation on peptide and APC stimulation (Soares et al, 2004). Further signalling studies showed that the expression of GRAIL in T cells inhibited RhoA GTPase activation, whereas Ras activation and MAP kinase signalling pathways remained unaffected, suggesting an alternative mechanism by which GRAIL inhibits T-cell signalling (Su et al, 2006).
Consistent with a potential role for E3 ubiquitin ligases in the anergy phenomenon, T cells overexpressing Egr2 or Egr3 have a high basal level of Cbl-b. Conversely, no increase in Cbl-b expression was seen on anergy induction in Egr3- deficient T cells (Safford et al, 2005). However, whether E3 ubiquitin ligases are involved in the induction compared with the maintenance of T-cell anergy is not yet clear, and how they relate to altered Ras activation in the anergic state has not been established. Recent experiments using a dominant negative Cbl adenoviral vector directly transduced into CAR transgenic peripheral T cells resulted in an augmentation of TCR-dependent IL-2 production (Zha & Gajewski, 2007). However, these T cells were still dependent on CD28 co-stimulation to produce IL-2, and were still susceptible to anergy induction (Zha et al, 2006). Future studies of these relationships might benefit from the generation of conditional knockout mice combined with Cre-mediated deletion of Cbl genes in the peripheral T-cell compartment, which will eliminate a potential effect contributed by altered thymic selection when the factors are absent throughout T-cell development. One potential point of intersection between E3 ligases and Ras is through their effects on phospholipase C-γ1 (PLC-γ1), which is an upstream regulator of Ras activation through the generation of DAG. Both Cbl-b and Itch have been reported to interact with PLC-γ1, leading to its ubiquitination and degradation (Heissmeyer et al, 2004; Jeon et al, 2004).
Areas of uncertainty
The definitive experiments proving an essential role for DGKs in T-cell anergy have not yet been performed. The use of pharmacological DGK inhibitors that augment the function of anergic T cells is subject to potential off-target effects of the compounds, and high doses can also result in cellular toxicity. A potential caveat of the use of genetic knockout mice deficient in DGK-α and DGK-ζ is the potential for subtle effects on thymic development, as well as the possible impact of DGK-deficiency in non-T cells. The importance of Ras signalling in thymic selection (Swan et al, 1995) suggests that T cells from DGK-deficient mice could be altered and perhaps emerge from the thymus being less susceptible to anergy, although no gross alterations in thymocyte phenotype have been observed (Olenchock et al, 2006). There is a potential for redundancy between DGK family members and so functional compensation might also occur in mice deficient in single DGK genes. For these reasons, conditionally targeted mice are being generated to allow for direct deletion of DGK-α and/or DGK-ζ in post-thymic T cells.
Whether increased DGK expression and defective Ras activation explain T-cell dysfunction in all models of T-cell anergy is also not clear. Models of in vivo adaptive tolerance have shown a defect in TCR-induced calcium flux (Chiodetti et al, 2006), which suggests the possibility of some differences in signalling deficits in various anergy models. However, it should be noted that T cells anergized in vitro also show a defect in calcium signalling (Gajewski et al, 1994b), but that a short rest period of 1–2 days after anergy induction results in recovery of a normal calcium flux but persistence of T-cell dysfunction (Gajewski et al, 1995). Therefore, there remains a need for careful comparative experiments investigating the biochemical and molecular alterations in anergic T cells using several in vitro and in vivo model systems, in which the cells are analysed and treated in similar ways.
Conclusions
The molecular alterations in anergic T cells have become clearer as a result of recently published experiments. A current working model suggests that excessive calcium/NFAT-based signalling leads to upregulated expression of Egr2 and/or Egr3, which in turn drives the expression of several negative regulators of TCR-based signalling. A crucial factor is DGK-α, which might function to deplete DAG and result in blunted RasGRP1-mediated Ras activation. Upregulated E3 ubiquitin ligases might also contribute to disrupted signalling through ubiquitination and either degradation or altered subcellular localization of other signalling intermediates. Further in-depth study of the regulation of T-cell anergy should lead to the development of pharmacological approaches either to promote the anergic state in settings of autoimmunity and transplantation, or to restore T-cell function in the settings of cancer and chronic infection.

Yan Zheng, Thomas F. Gajewski & Yuanyuan Zha
Acknowledgments
Work described in this review was supported by R21 AI59818 and P01 CA97296 grants from the National Institutes of Health, USA.
References
- Anandasabapathy N, Ford GS, Bloom D, Holness C, Paragas V, Seroogy C, Skrenta H, Hollenhorst M, Fathman CG, Soares L (2003) GRAIL: an E3 ubiquitin ligase that inhibits cytokine gene transcription is expressed in anergic CD4+ T cells. Immunity 18: 535–547 [DOI] [PubMed] [Google Scholar]
- Bachmaier K et al. (2000) Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 403: 211–216 [DOI] [PubMed] [Google Scholar]
- Beverly B, Kang SM, Lenardo MJ, Schwartz RH (1992) Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol 4: 661–671 [DOI] [PubMed] [Google Scholar]
- Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB (1995) CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity 3: 87–98 [DOI] [PubMed] [Google Scholar]
- Boussiotis VA, Freeman GJ, Berezovskaya A, Barber DL, Nadler LM (1997) Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science 278: 124–128 [DOI] [PubMed] [Google Scholar]
- Chai JG, Lechler RI (1997) Immobilized anti-CD3 mAb induces anergy in murine naive and memory CD4+ T cells in vitro. Int Immunol 9: 935–944 [DOI] [PubMed] [Google Scholar]
- Chavrier P, Zerial M, Lemaire P, Almendral J, Bravo R, Charnay P (1988) A gene encoding a protein with zinc fingers is activated during G0/G1 transition in cultured cells. EMBO J 7: 29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodetti L, Choi S, Barber DL, Schwartz RH (2006) Adaptive tolerance and clonal anergy are distinct biochemical states. J Immunol 176: 2279–2291 [DOI] [PubMed] [Google Scholar]
- Crespi D, Massa S, Basso V, Colombetti S, Mueller DL, Mondino A (2002) Constitutive active p21ras enhances primary T cell responsiveness to Ca2+ signals without interfering with the induction of clonal anergy. Eur J Immunol 32: 2500–2509 [DOI] [PubMed] [Google Scholar]
- Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, Stone JC (2000) RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol 1: 317–321 [DOI] [PubMed] [Google Scholar]
- Dubois PM, Pihlgren M, Tomkowiak M, Van Mechelen M, Marvel J (1998) Tolerant CD8 T cells induced by multiple injections of peptide antigen show impaired TCR signaling and altered proliferative responses in vitro and in vivo. J Immunol 161: 5260–5267 [PubMed] [Google Scholar]
- Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, Barry M, Bleakley RC, Ostergaard HL, Stone JC (2000) RasGRP links T-cell receptor signaling to Ras. Blood 95: 3199–3203 [PubMed] [Google Scholar]
- Essery G, Feldmann M, Lamb JR (1988) Interleukin-2 can prevent and reverse antigen-induced unresponsiveness in cloned human T lymphocytes. Immunology 64: 413–417 [PMC free article] [PubMed] [Google Scholar]
- Fang D, Elly C, Gao B, Fang N, Altman Y, Joazeiro C, Hunter T, Copeland N, Jenkins N, Liu YC (2002) Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat Immunol 3: 281–287 [DOI] [PubMed] [Google Scholar]
- Fields PE, Gajewski TF, Fitch FW (1996a) Blocked Ras activation in anergic CD4+ T cells. Science 271: 1276–1278 [DOI] [PubMed] [Google Scholar]
- Fields P, Fitch FW, Gajewski TF (1996b) Control of T lymphocyte signal transduction through clonal anergy. J Mol Med 74: 673–683 [DOI] [PubMed] [Google Scholar]
- Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB (2002) The CD28 signaling pathway regulates glucose metabolism. Immunity 16: 769–777 [DOI] [PubMed] [Google Scholar]
- Gajewski TF, Lancki DW, Stack R, Fitch FW (1994a) “Anergy” of TH0 helper T lymphocytes induces downregulation of TH1 characteristics and a transition to a TH2-like phenotype. J Exp Med 179: 481–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Qian D, Fields P, Fitch FW (1994b) Anergic T-lymphocyte clones have altered inositol phosphate, calcium, and tyrosine kinase signaling pathways. Proc Natl Acad Sci USA 91: 38–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Fields P, Fitch FW (1995) Induction of the increased Fyn kinase activity in anergic T helper type 1 clones requires calcium and protein synthesis and is sensitive to cyclosporin A. Eur J Immunol 25: 1836–1842 [DOI] [PubMed] [Google Scholar]
- Heissmeyer V, Macian F, Im SH, Varma R, Feske S, Venuprasad K, Gu H, Liu YC, Dustin ML, Rao A (2004) Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol 5: 255–265 [DOI] [PubMed] [Google Scholar]
- Jenkins MK, Schwartz RH (1987) Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med 165: 302–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon MS et al. (2004) Essential role of the E3 ubiquitin ligase Cbl-b in T cell anergy induction. Immunity 21: 167–177 [DOI] [PubMed] [Google Scholar]
- Kang SM, Beverly B, Tran AC, Brorson K, Schwartz RH, Lenardo MJ (1992) Transactivation by AP-1 is a molecular target of T cell clonal anergy. Science 257: 1134–1138 [DOI] [PubMed] [Google Scholar]
- Kawabe Y, Ochi A (1990) Selective anergy of V β 8+,CD4+ T cells in Staphylococcus enterotoxin B-primed mice. J Exp Med 172: 1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney ER, Pape KA, Loh DY, Jenkins MK (1994) Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity 1: 327–339 [DOI] [PubMed] [Google Scholar]
- Korb LC, Mirshahidi S, Ramyar K, Sadighi Akha AA, Sadegh-Nasseri S (1999) Induction of T cell anergy by low numbers of agonist ligands. J Immunol 162: 6401–6409 [PubMed] [Google Scholar]
- Li W, Whaley CD, Mondino A, Mueller DL (1996) Blocked signal transduction to the ERK and JNK protein kinases in anergic CD4+ T cells. Science 271: 1272–1276 [DOI] [PubMed] [Google Scholar]
- Lindstein T, June CH, Ledbetter JA, Stella G, Thompson CB (1989) Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science 244: 339–343 [DOI] [PubMed] [Google Scholar]
- Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A (2002) Transcriptional mechanisms underlying lymphocyte tolerance. Cell 109: 719–731 [DOI] [PubMed] [Google Scholar]
- Mirshahidi S, Huang C-T, Sadegh-Nasseri S (2001) Anergy in peripheral memory CD4+ T cells induced by low avidity engagement of T cell receptor. J Exp Med 194: 719–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor A, Campi G, Du G, Zheng Y, Foster DA, Dustin ML, Philips MR (2007) The lymphocyte function-associated antigen-1 receptor costimulates plasma membrane Ras via phospholipase D2. Nat Cell Biol 9: 713–719 [DOI] [PubMed] [Google Scholar]
- Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP (2006) Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol 7: 1174–1181 [DOI] [PubMed] [Google Scholar]
- Pape KA, Merica R, Mondino A, Khoruts A, Jenkins MK (1998) Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J Immunol 160: 4719–4729 [PubMed] [Google Scholar]
- Patwardhan S, Gashler A, Siegel MG, Chang LC, Joseph LJ, Shows TB, Le Beau MM, Sukhatme VP (1991) EGR3, a novel member of the Egr family of genes encoding immediate-early transcription factors. Oncogene 6: 917–928 [PubMed] [Google Scholar]
- Perry WL, Hustad CM, Swing DA, O'Sullivan TN, Jenkins NA, Copeland NG (1998) The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat Genet 18: 143–146 [DOI] [PubMed] [Google Scholar]
- Peterson AC, Marks RE, Fields PE, Imamoto A, Gajewski TF (2003) T cell development and function in CrkL-deficient mice. Eur J Immunol 33: 2687–2695 [DOI] [PubMed] [Google Scholar]
- Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A (2007) Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol Cell Biol 27: 2732–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safford M et al. (2005) Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol 6: 472–480 [DOI] [PubMed] [Google Scholar]
- Schwartz RH (2003) T cell anergy. Annu Rev Immunol 21: 305–334 [DOI] [PubMed] [Google Scholar]
- Sebzda E, Bracke M, Tugal T, Hogg N, Cantrell DA (2002) Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat Immunol 3: 251–258 [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J, Evavold BD, Allen PM (1993) Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature 363: 156–159 [DOI] [PubMed] [Google Scholar]
- Soares L, Seroogy C, Skrenta H, Anandasabapathy N, Lovelace P, Chung CD, Engleman E, Fathman CG (2004) Two isoforms of otubain 1 regulate T cell anergy via GRAIL. Nat Immunol 5: 45–54 [DOI] [PubMed] [Google Scholar]
- Su L, Lineberry N, Huh Y, Soares L, Fathman CG (2006) A novel E3 ubiquitin ligase substrate screen identifies Rho guanine dissociation inhibitor as a substrate of gene related to anergy in lymphocytes. J Immunol 177: 7559–7566 [DOI] [PubMed] [Google Scholar]
- Swan KA, Alberola-Ila J, Gross JA, Appleby MW, Forbush KA, Thomas JF, Perlmutter RM (1995) Involvement of p21ras distinguishes positive and negative selection in thymocytes. EMBO J 14: 276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telander DG, Malvey EN, Mueller DL (1999) Evidence for repression of IL-2 gene activation in anergic T cells. J Immunol 162: 1460–1465 [PubMed] [Google Scholar]
- Tognon CE, Kirk HE, Passmore LA, Whitehead IP, Der CJ, Kay RJ (1998) Regulation of RasGRP via a phorbol ester-responsive C1 domain. Mol Cell Biol 18: 6995–7008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YY, Leon RP, Marks R, Cham CM, Schaack J, Gajewski TF, DeGregori J (2000) Transgenic expression of the Coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells. Proc Natl Acad Sci USA 97: 13784–13789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wange RL (2000) LAT, the linker for activation of T cells: a bridge between T cell-specific and general signaling pathways. Sci STKE 2000: RE1. [DOI] [PubMed] [Google Scholar]
- Zha Y, Gajewski TF (2007) An adenoviral vector encoding dominant negative Cbl lowers the threshold for T cell activation in post-thymic T cells. Cell Immunol 247: 95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha Y, Marks R, Ho AW, Peterson AC, Janardhan S, Brown I, Praveen K, Stang S, Stone JC, Gajewski TF (2006) T cell anergy is reversed by active Ras and is regulated by diacylglycerol kinase-α. Nat Immunol 7: 1166–1173 [DOI] [PubMed] [Google Scholar]