Abstract
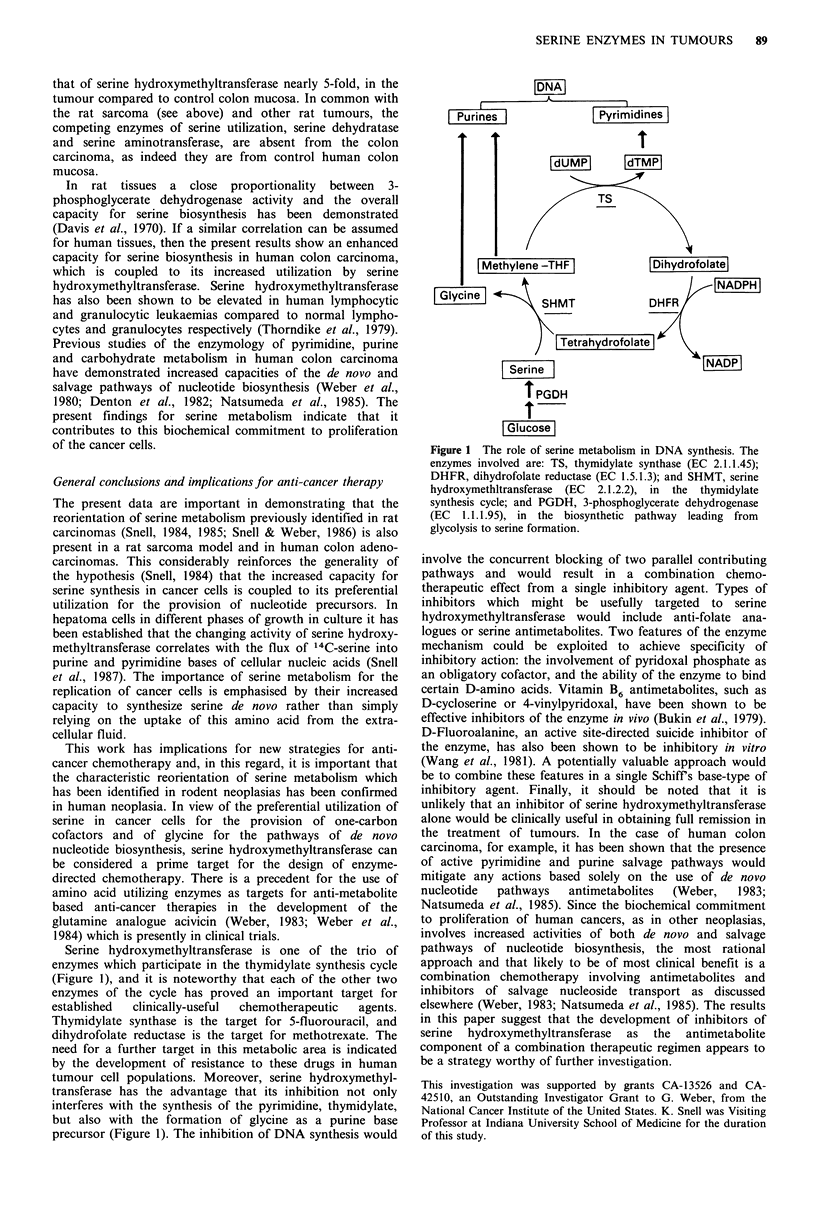
The activities of 3-phosphoglycerate dehydrogenase, an enzyme of serine biosynthesis, and serine hydroxymethyltransferase, serine dehydratase and serine aminotransferase, which are competing enzymes of serine utilization, were assayed in human colon carcinomas from patients and in transplantable rat sarcomas. Serine dehydratase and serine aminotransferase activities were absent, whereas 3-phosphoglycerate dehydrogenase and serine hydroxymethyltransferase activities were markedly increased in both tumour types. Serine hydroxymethyltransferase catalyses the formation of glycine and methylene tetrahydrofolate which are important precursors for nucleotide biosynthesis. The observed enzymic imbalance in these tumours ensures that an increased capacity for the synthesis of serine is coupled to its utilisation for nucleotide biosynthesis as a part of the biochemical commitment to cellular replication in cancer cells. That this pattern is found in sarcomas and carcinomas, and in tumours of human and rodent origin, signifies its universal importance for the biochemistry of the cancer cell and singles it out as a potential target site for anti-cancer chemotherapy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis J. L., Fallon H. J., Morris H. P. Two enzymes of serine metabolism in rat liver and hepatomas. Cancer Res. 1970 Dec;30(12):2917–2920. [PubMed] [Google Scholar]
- Denton J. E., Lui M. S., Aoki T., Sebolt J., Takeda E., Eble J. N., Glover J. L., Weber G. Enzymology of pyrimidine and carbohydrate metabolism in human colon carcinomas. Cancer Res. 1982 Mar;42(3):1176–1183. [PubMed] [Google Scholar]
- Eichler H. G., Hubbard R., Snell K. The role of serine hydroxymethyltransferase in cell proliferation: DNA synthesis from serine following mitogenic stimulation of lymphocytes. Biosci Rep. 1981 Feb;1(2):101–106. doi: 10.1007/BF01117006. [DOI] [PubMed] [Google Scholar]
- Harris B. A., Weigent D. A., Nelson J. A. Effects of 6-thiopurines on the transforming activity of bacillus subtilis dexoyribonucleic acid. Biochem Pharmacol. 1979 Apr 1;28(7):1169–1173. doi: 10.1016/0006-2952(79)90324-1. [DOI] [PubMed] [Google Scholar]
- Knox W. E., Herzfeld A., Hudson J. Phosphoserine phosphatase distribution in normal and neoplastic rat tissues. Arch Biochem Biophys. 1969 Jul;132(2):397–403. doi: 10.1016/0003-9861(69)90381-6. [DOI] [PubMed] [Google Scholar]
- Natsumeda Y., Lui M. S., Emrani J., Faderan M. A., Reardon M. A., Eble J. N., Glover J. L., Weber G. Purine enzymology of human colon carcinomas. Cancer Res. 1985 Jun;45(6):2556–2559. [PubMed] [Google Scholar]
- Popp M. B., Morrison S. D., Brennan M. F. Total parenteral nutrition in a methylcholanthrene-induced rat sarcoma model. Cancer Treat Rep. 1981;65 (Suppl 5):137–143. [PubMed] [Google Scholar]
- Rowe P. B., Sauer D., Fahey D., Craig G., McCairns E. One-carbon metabolism in lectin-activated human lymphocytes. Arch Biochem Biophys. 1985 Jan;236(1):277–288. doi: 10.1016/0003-9861(85)90627-7. [DOI] [PubMed] [Google Scholar]
- Snell K. Enzymes of serine metabolism in normal and neoplastic rat tissues. Biochim Biophys Acta. 1985 Dec 13;843(3):276–281. doi: 10.1016/0304-4165(85)90149-7. [DOI] [PubMed] [Google Scholar]
- Snell K. Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv Enzyme Regul. 1984;22:325–400. doi: 10.1016/0065-2571(84)90021-9. [DOI] [PubMed] [Google Scholar]
- Snell K., Natsumeda Y., Weber G. The modulation of serine metabolism in hepatoma 3924A during different phases of cellular proliferation in culture. Biochem J. 1987 Jul 15;245(2):609–612. doi: 10.1042/bj2450609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Weber G. Enzymic imbalance in serine metabolism in rat hepatomas. Biochem J. 1986 Jan 15;233(2):617–620. doi: 10.1042/bj2330617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike J., Pelliniemi T. T., Beck W. S. Serine hydroxymethyltransferase activity and serine incorporation in leukocytes. Cancer Res. 1979 Sep;39(9):3435–3440. [PubMed] [Google Scholar]
- Wang E. A., Kallen R., Walsh C. Mechanism-based inactivation of serine transhydroxymethylases by D-fluoroalanine and related amino acids. J Biol Chem. 1981 Jul 10;256(13):6917–6926. [PubMed] [Google Scholar]
- Weber G. Biochemical strategy of cancer cells and the design of chemotherapy: G. H. A. Clowes Memorial Lecture. Cancer Res. 1983 Aug;43(8):3466–3492. [PubMed] [Google Scholar]
- Weber G., Burt M. E., Jackson R. C., Prajda N., Lui M. S., Takeda E. Purine and pyrimidine enzymic programs and nucleotide pattern in sarcoma. Cancer Res. 1983 Mar;43(3):1019–1023. [PubMed] [Google Scholar]
- Weber G., Lui M. S., Takeda E., Denton J. E. Enzymology of human colon tumors. Life Sci. 1980 Sep 1;27(9):793–799. doi: 10.1016/0024-3205(80)90333-1. [DOI] [PubMed] [Google Scholar]
- Weber G., Natsumeda Y., Lui M. S., Faderan M. A., Liepnieks J. J., Elliott W. L. Control of enzymic programs and nucleotide pattern in cancer cells by acivicin and tiazofurin. Adv Enzyme Regul. 1984;22:69–93. doi: 10.1016/0065-2571(84)90009-8. [DOI] [PubMed] [Google Scholar]


