Abstract
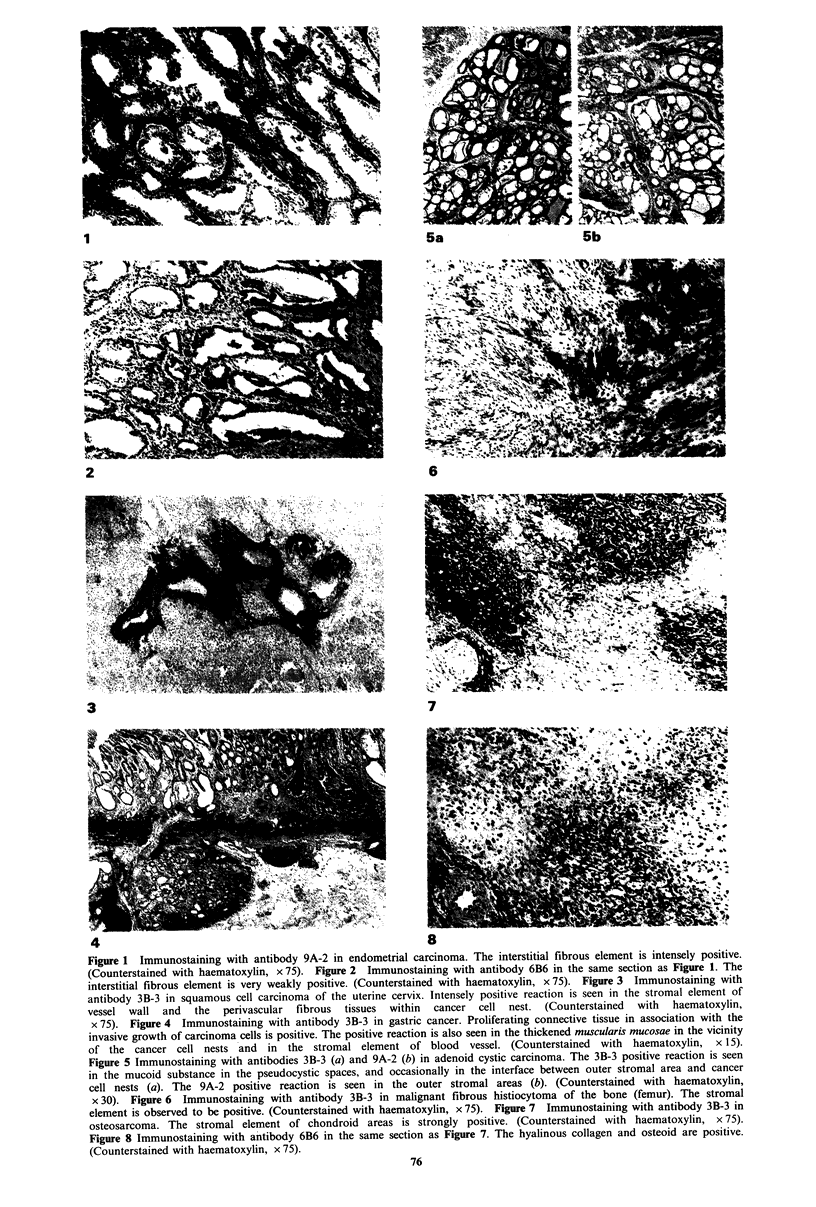
Immunohistochemical localization of chondroitin sulphate and dermatan sulphate proteoglycans (PGs) was observed in 70 tumour tissues, using monoclonal antibodies 9A-2 and 3B-3 raised against core molecules obtained from chondroitin sulphate PG by chondroitinase ABC-treatment. They recognize a stub of delta Di-4S and delta Di-6S binding to core protein via a linkage tetrasaccharide, respectively. The antibody 6B6 raised against dermatan sulphate PG obtained from an ovarian fibroma capsule in our laboratory was also used. The interstitial fibrous elements, so-called 'specific stroma' within the cancer cell nests contained chondroitin 4-sulphate PG as revealed with 9A-2, whereas the surrounding connective tissue and the preexisting fibrous connective tissue involved in the tumour growth consisted of dermatan sulphate PG with a considerable amount of chondroitin 4-sulphate PG. Chondroitin 6-sulphate PG as revealed with 3B-3 was located in the connective tissue proliferating from blood vessels and muscle tissue in association with the invasive growth of tumour cells. Chondroitin 6-sulphate PG was also observed in the basement membrane components of some tumours. In non-epithelial tumours (fibrogenic, chondrogenic, osteogenic and neurogenic tumours), chondroitin 4-sulphate was in fibrous portions. When collagenization and hyalinization progressed, dermatan sulphate PG was observed to increase in quantity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bano M., Zwiebel J. A., Salomon D. S., Kidwell W. R. Detection and partial characterization of collagen synthesis stimulating activities in rat mammary adenocarcinomas. J Biol Chem. 1983 Feb 25;258(4):2729–2735. [PubMed] [Google Scholar]
- Carrino D. A., Caplan A. I. Isolation and partial characterization of high-buoyant-density proteoglycans synthesized in ovo by embryonic chick skeletal muscle and heart. J Biol Chem. 1984 Oct 25;259(20):12419–12430. [PubMed] [Google Scholar]
- Carrino D. A., Caplan A. I. Isolation and preliminary characterization of proteoglycans synthesized by skeletal muscle. J Biol Chem. 1982 Dec 10;257(23):14145–14154. [PubMed] [Google Scholar]
- Castor C. W., Miller J. W., Walz D. A. Structural and biological characteristics of connective tissue activating peptide (CTAP-III), a major human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1983 Feb;80(3):765–769. doi: 10.1073/pnas.80.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R., Couchman J. R. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc. 1985 Feb;44(2):386–393. [PubMed] [Google Scholar]
- Couchman J. R., Caterson B., Christner J. E., Baker J. R. Mapping by monoclonal antibody detection of glycosaminoglycans in connective tissues. Nature. 1984 Feb 16;307(5952):650–652. doi: 10.1038/307650a0. [DOI] [PubMed] [Google Scholar]
- Damle S. P., Cöster L., Gregory J. D. Proteodermatan sulfate isolated from pig skin. J Biol Chem. 1982 May 25;257(10):5523–5527. [PubMed] [Google Scholar]
- Dietrich C. P., Sampaio L. O., Toledo O. M., Cássaro C. M. Cell recognition and adhesiveness: a possible biological role for the sulfated mucopolysaccharides. Biochem Biophys Res Commun. 1977 Mar 21;75(2):329–336. doi: 10.1016/0006-291x(77)91046-4. [DOI] [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. Angiogenesis in vitro. Nature. 1980 Dec 11;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Franzén A., Heinegård D. Characterization of proteoglycans from the calcified matrix of bovine bone. Biochem J. 1984 Nov 15;224(1):59–66. doi: 10.1042/bj2240059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann J., Thiel J., Schmidt A., Buddecke E. Increased activity of chondroitin sulfate-synthesizing enzymes during proliferation of arterial smooth muscle cells. Exp Cell Res. 1986 Dec;167(2):484–494. doi: 10.1016/0014-4827(86)90188-6. [DOI] [PubMed] [Google Scholar]
- Hübner G., Klein H. J., Kleinsasser O., Schiefer H. G. Role of myoepithelial cells in the development of salivary gland tumors. Cancer. 1971 May;27(5):1255–1261. doi: 10.1002/1097-0142(197105)27:5<1255::aid-cncr2820270533>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986 Mar 25;261(9):4337–4345. [PubMed] [Google Scholar]
- Iozzo R. V., Bolender R. P., Wight T. N. Proteoglycan changes in the intercellular matrix of human colon carcinoma: an integrated biochemical and stereologic analysis. Lab Invest. 1982 Aug;47(2):124–138. [PubMed] [Google Scholar]
- Iozzo R. V. Proteoglycans and neoplastic--mesenchymal cell interactions. Hum Pathol. 1984 Jan;15(1):2–10. doi: 10.1016/s0046-8177(84)80326-3. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V. Proteoglycans: structure, function, and role in neoplasia. Lab Invest. 1985 Oct;53(4):373–396. [PubMed] [Google Scholar]
- Iozzo R. V., Wight T. N. Isolation and characterization of proteoglycans synthesized by human colon and colon carcinoma. J Biol Chem. 1982 Sep 25;257(18):11135–11144. [PubMed] [Google Scholar]
- Kojima J., Nakamura N., Kanatani M., Omori K. The glycosaminoglycans in human hepatic cancer. Cancer Res. 1975 Mar;35(3):542–547. [PubMed] [Google Scholar]
- Oike Y., Kimata K., Shinomura T., Nakazawa K., Suzuki S. Structural analysis of chick-embryo cartilage proteoglycan by selective degradation with chondroitin lyases (chondroitinases) and endo-beta-D-galactosidase (keratanase). Biochem J. 1980 Oct 1;191(1):193–207. doi: 10.1042/bj1910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L. C., Choi H. U., Tang L. H., Johnson T. L., Pal S., Webber C., Reiner A., Poole A. R. Isolation of dermatan sulfate proteoglycans from mature bovine articular cartilages. J Biol Chem. 1985 May 25;260(10):6304–6313. [PubMed] [Google Scholar]
- Scott J. E., Haigh M. 'Small'-proteoglycan:collagen interactions: keratan sulphate proteoglycan associates with rabbit corneal collagen fibrils at the 'a' and 'c' bands. Biosci Rep. 1985 Sep;5(9):765–774. doi: 10.1007/BF01119875. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Proteoglycan-collagen interactions in intervertebral disc. A chondroitin sulphate proteoglycan associates with collagen fibrils in rabbit annulus fibrosus at the d-e bands. Biosci Rep. 1986 Oct;6(10):879–888. doi: 10.1007/BF01116241. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Proteoglycan-type I collagen fibril interactions in bone and non-calcifying connective tissues. Biosci Rep. 1985 Jan;5(1):71–81. doi: 10.1007/BF01117443. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Orford C. R. Dermatan sulphate-rich proteoglycan associates with rat tail-tendon collagen at the d band in the gap region. Biochem J. 1981 Jul 1;197(1):213–216. doi: 10.1042/bj1970213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue M., Miura K., Kataoka K., Tsuji K., Takeuchi J. Glycosaminoglycan-synthetic activity of neoplastic and non-neoplastic adipose tissues. Br J Cancer. 1980 Sep;42(3):477–480. doi: 10.1038/bjc.1980.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue M., Takeuchi J., Miura K., Kawase K., Mizuno F., Sato E. Glycosaminoglycan content and synthesis in gastric carcinoma. Br J Cancer. 1980 Jul;42(1):78–84. doi: 10.1038/bjc.1980.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue M., Takeuchi J., Yoshida K., Akao S., Fukatsu T., Nagasaka T., Nakashima N. Isolation and characterization of proteoglycans from human nonepithelial tumors. Cancer Res. 1987 Jan 1;47(1):160–168. [PubMed] [Google Scholar]
- Takeuchi J. Growth-promoting effect of chondroitin sulphate on solid Ehrlich ascites tumour. Nature. 1965 Jul 31;207(996):537–538. doi: 10.1038/207537b0. [DOI] [PubMed] [Google Scholar]
- Takeuchi J., Sobue M., Sato E., Shamoto M., Miura K. Variation in glycosaminoglycan components of breast tumors. Cancer Res. 1976 Jul;36(7 Pt 1):2133–2139. [PubMed] [Google Scholar]
- Toida M., Takeuchi J., Sobue M., Tsukidate K., Akao S., Fukatsu T., Nakashima N. Histochemical studies on pseudocysts in adenoid cystic carcinoma of the human salivary gland. Histochem J. 1985 Aug;17(8):913–924. doi: 10.1007/BF01004186. [DOI] [PubMed] [Google Scholar]
- Uldbjerg N., Malmström A., Ekman G., Sheehan J., Ulmsten U., Wingerup L. Isolation and characterization of dermatan sulphate proteoglycan from human uterine cervix. Biochem J. 1983 Feb 1;209(2):497–503. doi: 10.1042/bj2090497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel K. G., Heinegård D. Characterization of proteoglycans from adult bovine tendon. J Biol Chem. 1985 Aug 5;260(16):9298–9306. [PubMed] [Google Scholar]










