Abstract
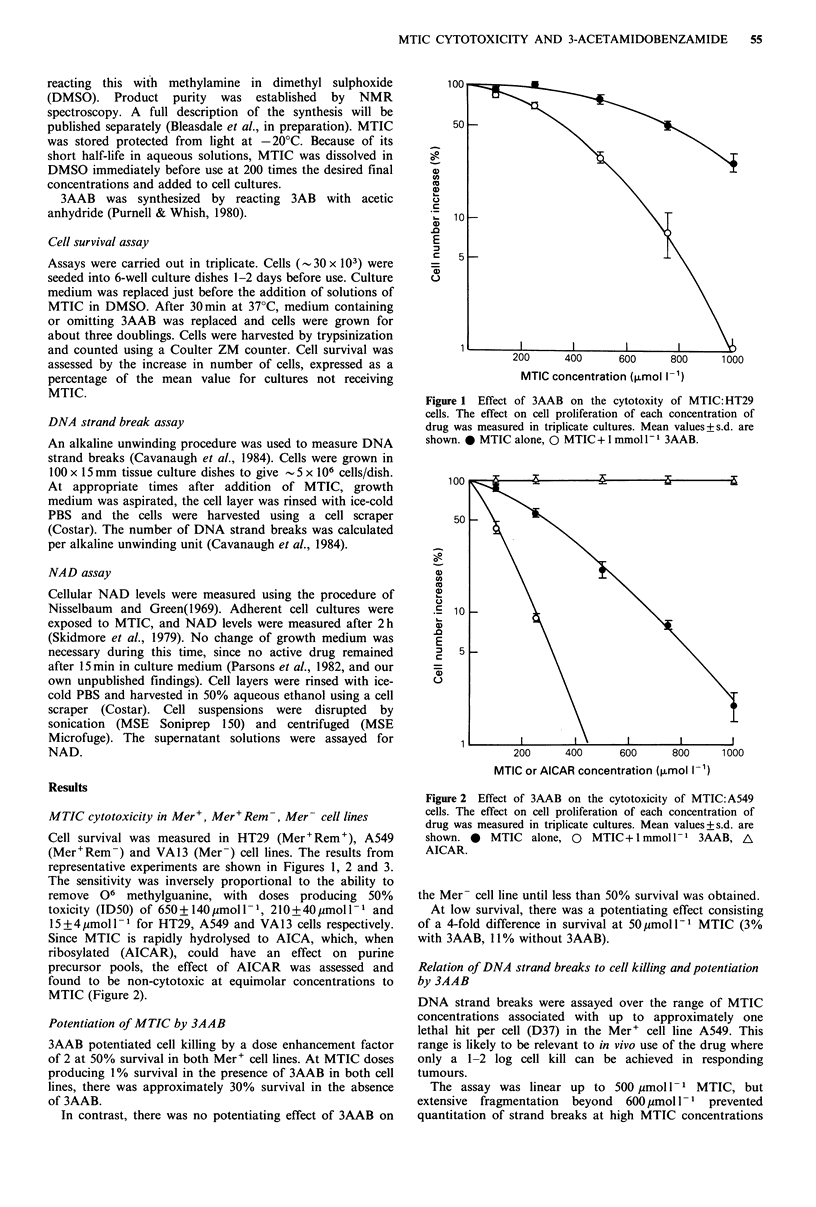
Mechanisms of resistance to the active metabolite 5-(3-methyl-1-triazeno)imidazole-4-carboxamide (MTIC) of the drug 5-(3,3-dimethyl-1-triázeno)imidazole-4-carboxamide (DTIC) were studied in three human cell lines with differing amounts of the repair enzyme O6-alkylguanine-DNA alkyltransferase (O6AT). The lines were HT29 (Mer+Rem+), A549 (Mer+Rem-) and VA13 (Mer-). The ability to repair O6 methyl-guanine was directly related to resistance to MTIC (HT29 ID50 650 mumol l-1, A549 ID50 210 mumol l-1, VA13 ID50 15 mumol l-1. MTIC produced DNA single strand breaks over the range of one log of cell kill, but depletion of cellular NAD levels could not be detected until there was greater than 95% cell kill. Inhibitors of the repair enzyme adenosine diphosphoribosyl transferase (ADPRT) potentiated killing by 2-fold in the Mer+ cell lines but not the Mer- line. The enhancement was directly proportional to an increase in DNA strand breaks but not a change in their half-life. Therefore resistance to the clinically used methylating agent MTIC can be partly overcome by inhibiting ADPRT but a role for ADPRT as a suicide mechanism in response to alkylating agent damage is unlikely.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carson D. A., Seto S., Wasson D. B., Carrera C. J. DNA strand breaks, NAD metabolism, and programmed cell death. Exp Cell Res. 1986 Jun;164(2):273–281. doi: 10.1016/0014-4827(86)90028-5. [DOI] [PubMed] [Google Scholar]
- Cavanaugh P. F., Jr, Pavelic Z. P., Porter C. W. Enhancement of 1,3-bis(2-chloroethyl)-1-nitrosourea-induced cytotoxicity and DNA damage by alpha-difluoromethylornithine in L1210 leukemia cells. Cancer Res. 1984 Sep;44(9):3856–3861. [PubMed] [Google Scholar]
- Cleaver J. E., Morgan W. F. Poly(ADP-ribose) synthesis is involved in the toxic effects of alkylating agents but does not regulate DNA repair. Mutat Res. 1985 Jun-Jul;150(1-2):69–76. doi: 10.1016/0027-5107(85)90102-2. [DOI] [PubMed] [Google Scholar]
- Comis R. L. DTIC (NSC-45388) in malignant melanoma: a perspective. Cancer Treat Rep. 1976 Feb;60(2):165–176. [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H., Scudiero D. A., Meyer S. A., Lubiniecki A. S., Girardi A. J., Galloway S. M., Bynum G. D. Defective repair of alkylated DNA by human tumour and SV40-transformed human cell strains. Nature. 1980 Dec 25;288(5792):724–727. doi: 10.1038/288724a0. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Erickson L. C., Laurent G., Sharkey N. A., Kohn K. W. DNA cross-linking and monoadduct repair in nitrosourea-treated human tumour cells. Nature. 1980 Dec 25;288(5792):727–729. doi: 10.1038/288727a0. [DOI] [PubMed] [Google Scholar]
- Gerson S. L., Miller K., Berger N. A. O6 alkylguanine-DNA alkyltransferase activity in human myeloid cells. J Clin Invest. 1985 Dec;76(6):2106–2114. doi: 10.1172/JCI112215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson N. W., Hartley J., La France R. J., Vaughan K. Differential cytotoxicity and DNA-damaging effects produced in human cells of the Mer+ and Mer- phenotypes by a series of alkyltriazenylimidazoles. Carcinogenesis. 1986 Feb;7(2):259–265. doi: 10.1093/carcin/7.2.259. [DOI] [PubMed] [Google Scholar]
- Gottlieb J. A., Benjamin R. S., Baker L. H., O'Bryan R. M., Sinkovics J. G., Hoogstraten B., Quagliana J. M., Rivkin S. E., Bodey G. P., Sr, Rodriguez V. Role of DTIC (NSC-45388) in the chemotherapy of sarcomas. Cancer Treat Rep. 1976 Feb;60(2):199–203. [PubMed] [Google Scholar]
- Grafstrom R. C., Pegg A. E., Trump B. F., Harris C. C. O6-alkylguanine-DNA alkyltransferase activity in normal human tissues and cells. Cancer Res. 1984 Jul;44(7):2855–2857. [PubMed] [Google Scholar]
- Harris A. L., Karran P., Lindahl T. O6-Methylguanine-DNA methyltransferase of human lymphoid cells: structural and kinetic properties and absence in repair-deficient cells. Cancer Res. 1983 Jul;43(7):3247–3252. [PubMed] [Google Scholar]
- Hayward I. P., Parsons P. G. Comparison of virus reactivation, DNA base damage, and cell cycle effects in autologous human melanoma cells resistant to methylating agents. Cancer Res. 1984 Jan;44(1):55–58. [PubMed] [Google Scholar]
- Hayward I. P., Parsons P. G. Epigenetic effects of the methylating agent 5-(3-methyl-1-triazeno) imidazole-4-carboxamide in human melanoma cells. Aust J Exp Biol Med Sci. 1984 Oct;62(Pt 5):597–606. doi: 10.1038/icb.1984.57. [DOI] [PubMed] [Google Scholar]
- Karran P., Williams S. A. The cytotoxic and mutagenic effects of alkylating agents on human lymphoid cells are caused by different DNA lesions. Carcinogenesis. 1985 May;6(5):789–792. doi: 10.1093/carcin/6.5.789. [DOI] [PubMed] [Google Scholar]
- Meer L., Janzer R. C., Kleihues P., Kolar G. F. In vivo metabolism and reaction with DNA of the cytostatic agent, 5-(3,3-dimethyl-1-triazeno)imidazole-4-carboxamide (DTIC). Biochem Pharmacol. 1986 Oct 1;35(19):3243–3247. doi: 10.1016/0006-2952(86)90419-3. [DOI] [PubMed] [Google Scholar]
- Moran M. F., Ebisuzaki K. Inhibition of poly(ADP-ribose)polymerase causes increased DNA strand breaks without decreasing strand rejoining in alkylated HeLa cells. FEBS Lett. 1985 Oct 14;190(2):279–282. doi: 10.1016/0014-5793(85)81300-4. [DOI] [PubMed] [Google Scholar]
- Myrnes B., Eggset G., Volden G., Krokan H. Enzymatic repair of premutagenic DNA lesions in human epidermis. Quantitation of O6-methylguanine-DNA methyltransferase and uracil-DNA glycosylase activities. Mutat Res. 1984 Mar-Apr;131(3-4):183–186. doi: 10.1016/0167-8817(84)90059-2. [DOI] [PubMed] [Google Scholar]
- Myrnes B., Norstrand K., Giercksky K. E., Sjunneskog C., Krokan H. A simplified assay for O6-methylguanine-DNA methyltransferase activity and its application to human neoplastic and non-neoplastic tissues. Carcinogenesis. 1984 Aug;5(8):1061–1064. doi: 10.1093/carcin/5.8.1061. [DOI] [PubMed] [Google Scholar]
- Nisselbaum J. S., Green S. A simple ultramicro method for determination of pyridine nucleotides in tissues. Anal Biochem. 1969 Feb;27(2):212–217. doi: 10.1016/0003-2697(69)90025-6. [DOI] [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- Parsons P. G., Smellie S. G., Morrison L. E., Hayward I. P. Properties of human melanoma cells resistant to 5-(3',3'-dimethyl-1-triazeno)imidazole-4-carboxamide and other methylating agents. Cancer Res. 1982 Apr;42(4):1454–1461. [PubMed] [Google Scholar]
- Preussmann R., von Hodenberg A. Mechanism of carcinogenesis with 1-aryl-3,3-dialkyltriazenes. II. In vitro-alkylation of guanosine, RNA and DNA with aryl-monoalkyltriazenes to form 7-alkylguanine. Biochem Pharmacol. 1970 Apr;19(4):1505–1508. doi: 10.1016/0006-2952(70)90068-7. [DOI] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scudiero D. A., Meyer S. A., Clatterbuck B. E., Mattern M. R., Ziolkowski C. H., Day R. S., 3rd Sensitivity of human cell strains having different abilities to repair O6-methylguanine in DNA to inactivation by alkylating agents including chloroethylnitrosoureas. Cancer Res. 1984 Jun;44(6):2467–2474. [PubMed] [Google Scholar]
- Sims J. L., Berger S. J., Berger N. A. Poly(ADP-ribose) Polymerase inhibitors preserve nicotinamide adenine dinucleotide and adenosine 5'-triphosphate pools in DNA-damaged cells: mechanism of stimulation of unscheduled DNA synthesis. Biochemistry. 1983 Oct 25;22(22):5188–5194. doi: 10.1021/bi00291a019. [DOI] [PubMed] [Google Scholar]
- Skidmore C. J., Davies M. I., Goodwin P. M., Halldorsson H., Lewis P. J., Shall S., Zia'ee A. A. The involvement of poly(ADP-ribose) polymerase in the degradation of NAD caused by gamma-radiation and N-methyl-N-nitrosourea. Eur J Biochem. 1979 Nov 1;101(1):135–142. doi: 10.1111/j.1432-1033.1979.tb04225.x. [DOI] [PubMed] [Google Scholar]
- Stevens M. F., Hickman J. A., Stone R., Gibson N. W., Baig G. U., Lunt E., Newton C. G. Antitumor imidazotetrazines. 1. Synthesis and chemistry of 8-carbamoyl-3-(2-chloroethyl)imidazo[5,1-d]-1,2,3,5-tetrazin-4(3 H)-one , a novel broad-spectrum antitumor agent. J Med Chem. 1984 Feb;27(2):196–201. doi: 10.1021/jm00368a016. [DOI] [PubMed] [Google Scholar]
- Tisdale M. J. Antitumour imidazotetrazines--XI: Effect of 8-carbamoyl-3-methylimidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one [CCRG 81045; M and B 39831 NSC 362856] on poly(ADP-ribose) metabolism. Br J Cancer. 1985 Nov;52(5):789–792. doi: 10.1038/bjc.1985.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbenhauer D., Wild C. P., Montesano R., Saffhill R., Boyle J. M., Huh N., Kirstein U., Thomale J., Rajewsky M. F., Lu S. H. O(6)-methyldeoxyguanosine in oesophageal DNA among individuals at high risk of oesophageal cancer. Int J Cancer. 1985 Dec 15;36(6):661–665. doi: 10.1002/ijc.2910360607. [DOI] [PubMed] [Google Scholar]
- Waldstein E. A., Cao E. H., Miller M. E., Cronkite E. P., Setlow R. B. Extracts of chronic lymphocytic leukemia lymphocytes have a high level of DNA repair activity fo O6-methylguanine. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4786–4790. doi: 10.1073/pnas.79.15.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker I. G., Th'ng J. P., Schrader T. J., Norry T. W. 3-Aminobenzamide does not increase repair patch size in mammalian cells. Can J Biochem Cell Biol. 1984 Jun;62(6):329–334. doi: 10.1139/o84-046. [DOI] [PubMed] [Google Scholar]
- Wani A. A., Wani G., D'Ambrosio S. M. Repair of DNA O-alkylation damage by various human organs. Biochem Biophys Res Commun. 1985 Dec 17;133(2):589–597. doi: 10.1016/0006-291x(85)90946-5. [DOI] [PubMed] [Google Scholar]
- Wiestler O., Kleihues P., Pegg A. E. O6-alkylguanine-DNA alkyltransferase activity in human brain and brain tumors. Carcinogenesis. 1984 Jan;5(1):121–124. doi: 10.1093/carcin/5.1.121. [DOI] [PubMed] [Google Scholar]
- Wilman D. E., Cox P. J., Goddard P. M., Hart L. I., Merai K., Newell D. R. Tumor inhibitory triazenes. 3. Dealkylation within an homologous series and its relation to antitumor activity. J Med Chem. 1984 Jul;27(7):870–874. doi: 10.1021/jm00373a011. [DOI] [PubMed] [Google Scholar]
- Wintersberger U., Wintersberger E. Poly ADP-ribosylation--a cellular emergency reaction? FEBS Lett. 1985 Sep 2;188(2):189–191. doi: 10.1016/0014-5793(85)80369-0. [DOI] [PubMed] [Google Scholar]


