Abstract
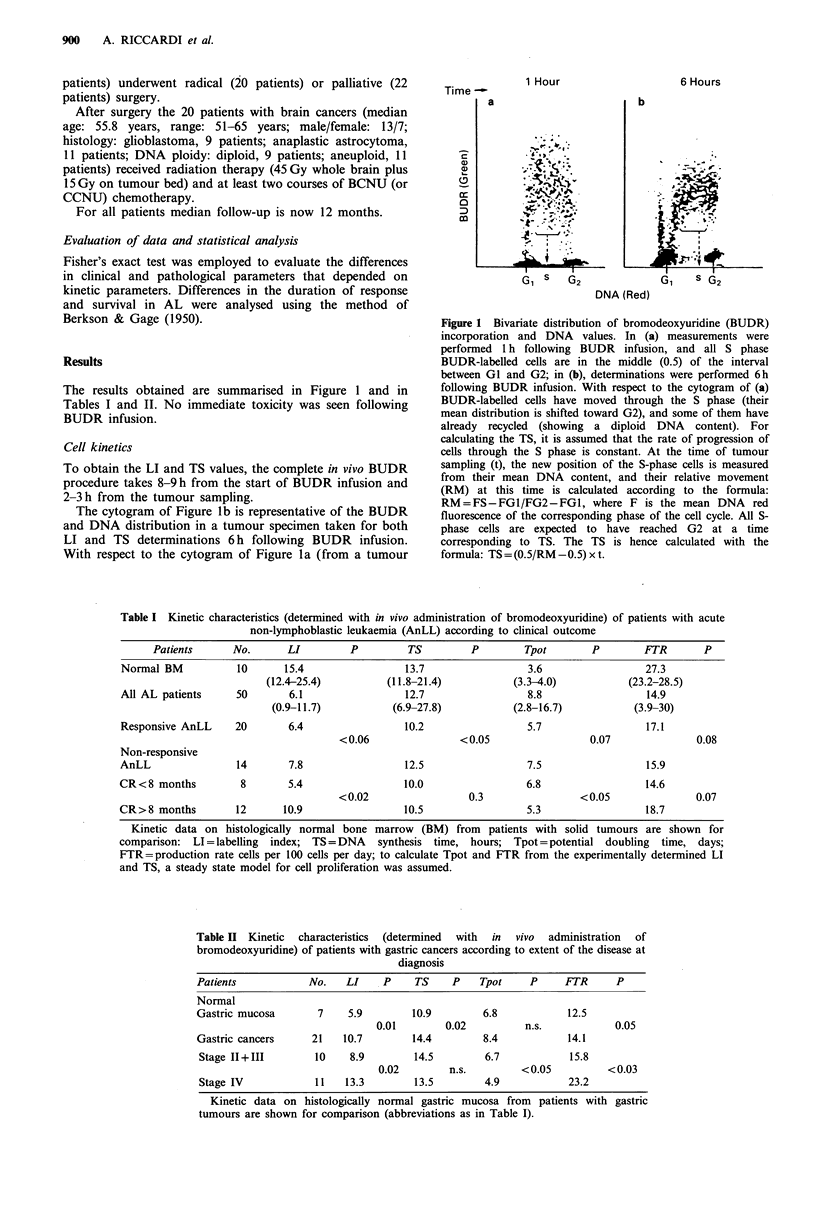
During a 15-month period, we used in vivo bromodeoxyuridine (BUDR) infusion to study cell kinetics in 112 consecutive patients with various types of malignant tumours: acute leukaemia (50 patients), gastric cancer (42) and brain gliomas (20). The in vivo BUDR method requires that a single tumour sample be taken 4-6 h after infusion and that bivariate flow cytometry (FCM) be employed to measure simultaneously the percentage of BUDR-labelled cells (which are identified with a green fluorescent anti-BUDR monoclonal antibody) and their mean DNA content (following propidium iodide staining). This technique rapidly furnishes the labelling index (LI) and the DNA synthesis time (TS), from which the tumour potential doubling time (Tpot) and production rate (fractional turnover rate, FTR) are calculated. The procedure took 6-9 h to complete and there was no immediate toxicity from BUDR administration. Successful LI and TS determinations were obtained in 89 (80%) and 80 (72%) of the 112 patients, respectively. Correlations were sought between kinetic parameters and a number of pathological and clinical ones. In 34 patients with acute non-lymphoblastic leukaemias who were uniformly treated for remission (CR) induction and maintenance, proliferative activity, as measured by Tpot and FTR, was greater in responsive than in non-responsive patients, and in those who experienced CR for over 8 months than in those who had a shorter CR. Proliferative activity was also greater in patients with advanced gastric cancers than in those with more limited disease. No correlations between kinetic and clinical and pathological parameters were found in gliomas. These data indicate the in vivo BUDR infusion coupled with FCM measurements can be performed in clinical settings to obtain kinetic data rapidly in quite large patient series. This will probably allow the inclusion of kinetic data in clinical trials aimed at evaluating the prognostic relevance of these data.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERKSON J., GAGE R. P. Calculation of survival rates for cancer. Proc Staff Meet Mayo Clin. 1950 May 24;25(11):270–286. [PubMed] [Google Scholar]
- Bauer K. D., Merkel D. E., Winter J. N., Marder R. J., Hauck W. W., Wallemark C. B., Williams T. J., Variakojis D. Prognostic implications of ploidy and proliferative activity in diffuse large cell lymphomas. Cancer Res. 1986 Jun;46(6):3173–3178. [PubMed] [Google Scholar]
- Begg A. C., McNally N. J., Shrieve D. C., Kärcher H. A method to measure the duration of DNA synthesis and the potential doubling time from a single sample. Cytometry. 1985 Nov;6(6):620–626. doi: 10.1002/cyto.990060618. [DOI] [PubMed] [Google Scholar]
- Cho K. G., Hoshino T., Nagashima T., Murovic J. A., Wilson C. B. Prediction of tumor doubling time in recurrent meningiomas. Cell kinetics studies with bromodeoxyuridine labeling. J Neurosurg. 1986 Dec;65(6):790–794. doi: 10.3171/jns.1986.65.6.0790. [DOI] [PubMed] [Google Scholar]
- Danova M., Riccardi A., Brugnatelli S., Fiocca R., Girino M., Villani L., Giordano P., Dionigi P., Giordano M., Buttini R. In vivo bromodeoxyuridine incorporation in human gastric cancer: a study on formalin-fixed and paraffin-embedded sections. Histochem J. 1988 Mar;20(3):125–130. doi: 10.1007/BF01746675. [DOI] [PubMed] [Google Scholar]
- Danova M., Riccardi A., Gaetani P., Wilson G. D., Mazzini G., Brugnatelli S., Buttini R., Butti G., Ucci G., Paoletti P. Cell kinetics of human brain tumors: in vivo study with bromodeoxyuridine and flow cytometry. Eur J Cancer Clin Oncol. 1988 May;24(5):873–880. doi: 10.1016/0277-5379(88)90196-4. [DOI] [PubMed] [Google Scholar]
- Danova M., Wilson G., Riccardi A., Mazzini G., Ucci G., Giordano M., Brugnatelli S., Luoni R., McNally N. J., Ascari E. In vivo administration of bromodeoxyuridine and flow cytometry for cell kinetic studies in human malignancies. Haematologica. 1987 Mar-Apr;72(2):115–119. [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Nagashima T., Cho K. G., Murovic J. A., Hodes J. E., Wilson C. B., Edwards M. S., Pitts L. H. S-phase fraction of human brain tumors in situ measured by uptake of bromodeoxyuridine. Int J Cancer. 1986 Sep 15;38(3):369–374. doi: 10.1002/ijc.2910380311. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Nagashima T., Murovic J. A., Wilson C. B., Davis R. L. Proliferative potential of human meningiomas of the brain. A cell kinetics study with bromodeoxyuridine. Cancer. 1986 Oct 1;58(7):1466–1472. doi: 10.1002/1097-0142(19861001)58:7<1466::aid-cncr2820580715>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Nagashima T., Murovic J., Levin E. M., Levin V. A., Rupp S. M. Cell kinetic studies of in situ human brain tumors with bromodeoxyuridine. Cytometry. 1985 Nov;6(6):627–632. doi: 10.1002/cyto.990060619. [DOI] [PubMed] [Google Scholar]
- Montecucco C., Riccardi A., Traversi E., Giordano P., Mazzini G., Ascari E. Proliferative activity of bone marrow cells in primary dysmyelopoietic (preleukemic) syndromes. Cancer. 1983 Oct 1;52(7):1190–1195. doi: 10.1002/1097-0142(19831001)52:7<1190::aid-cncr2820520711>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Moran R., Darzynkiewicz Z., Staiano-Coico L., Melamed M. R. Detection of 5-bromodeoxyuridine (BrdUrd) incorporation by monoclonal antibodies: role of the DNA denaturation step. J Histochem Cytochem. 1985 Aug;33(8):821–827. doi: 10.1177/33.8.3860561. [DOI] [PubMed] [Google Scholar]
- Murovic J. A., Nagashima T., Hoshino T., Edwards M. S., Davis R. L. Pediatric central nervous system tumors: a cell kinetic study with bromodeoxyuridine. Neurosurgery. 1986 Dec;19(6):900–904. doi: 10.1227/00006123-198612000-00003. [DOI] [PubMed] [Google Scholar]
- Nagashima T., Murovic J. A., Hoshino T., Wilson C. B., DeArmond S. J. The proliferative potential of human pituitary tumors in situ. J Neurosurg. 1986 Apr;64(4):588–593. doi: 10.3171/jns.1986.64.4.0588. [DOI] [PubMed] [Google Scholar]
- Paietta E., Mittermayer K., Schwarzmeier J. Proliferation kinetics and cyclic AMP as prognostic factors in adult acute leukemia. Cancer. 1980 Jul 1;46(1):102–108. doi: 10.1002/1097-0142(19800701)46:1<102::aid-cncr2820460118>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Raza A., Maheshwari Y., Preisler H. D. Differences in cell cycle characteristics among patients with acute nonlymphocytic leukemia. Blood. 1987 Jun;69(6):1647–1653. [PubMed] [Google Scholar]
- Raza A., Ucar K., Preisler H. D. Double labeling and in vitro versus in vivo incorporation of bromodeoxyuridine in patients with acute nonlymphocytic leukemia. Cytometry. 1985 Nov;6(6):633–640. doi: 10.1002/cyto.990060620. [DOI] [PubMed] [Google Scholar]
- Riccardi A., Danova M., Wilson G., Ucci G., Dörmer P., Mazzini G., Brugnatelli S., Girino M., McNally N. J., Ascari E. Cell kinetics in human malignancies studied with in vivo administration of bromodeoxyuridine and flow cytometry. Cancer Res. 1988 Nov 1;48(21):6238–6245. [PubMed] [Google Scholar]
- Riccardi A., Martinotti A., Perugini S. Cytokinetic studies in two cases of plasma cell leukemia. Haematologica. 1977 Dec;62(6):581–589. [PubMed] [Google Scholar]
- Riccardi A., Montecucco C. M., Danova M., Ucci G., Mazzini G., Giordano P. A., Pasquali F. Flow cytometric evaluation of proliferative activity and ploidy in myelodysplastic syndromes and acute leukemias. Basic Appl Histochem. 1986;30(2):181–192. [PubMed] [Google Scholar]
- Silvestrini R., Daidone M. G., Gasparini G. Cell kinetics as a prognostic marker in node-negative breast cancer. Cancer. 1985 Oct 15;56(8):1982–1987. doi: 10.1002/1097-0142(19851015)56:8<1982::aid-cncr2820560816>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Silvestrini R., Piazza R., Riccardi A., Rilke F. Correlation of cell kinetic findings with morphology of non-Hodgkin's malignant lymphomas. J Natl Cancer Inst. 1977 Mar;58(3):499–504. doi: 10.1093/jnci/58.3.499. [DOI] [PubMed] [Google Scholar]
- Ucci G., Riccardi A., Danova M., Montecucco C. M., Ascari E. In vitro evaluation of multiple kinetic parameters in human leukemia by quantitative 14-C autoradiography. Haematologica. 1985 Mar-Apr;70(2):101–105. [PubMed] [Google Scholar]
- Wilson G. D., McNally N. J., Dunphy E., Kärcher H., Pfragner R. The labelling index of human and mouse tumours assessed by bromodeoxyuridine staining in vitro and in vivo and flow cytometry. Cytometry. 1985 Nov;6(6):641–647. doi: 10.1002/cyto.990060621. [DOI] [PubMed] [Google Scholar]
- deFazio A., Leary J. A., Hedley D. W., Tattersall M. H. Immunohistochemical detection of proliferating cells in vivo. J Histochem Cytochem. 1987 May;35(5):571–577. doi: 10.1177/35.5.3549891. [DOI] [PubMed] [Google Scholar]


