Abstract
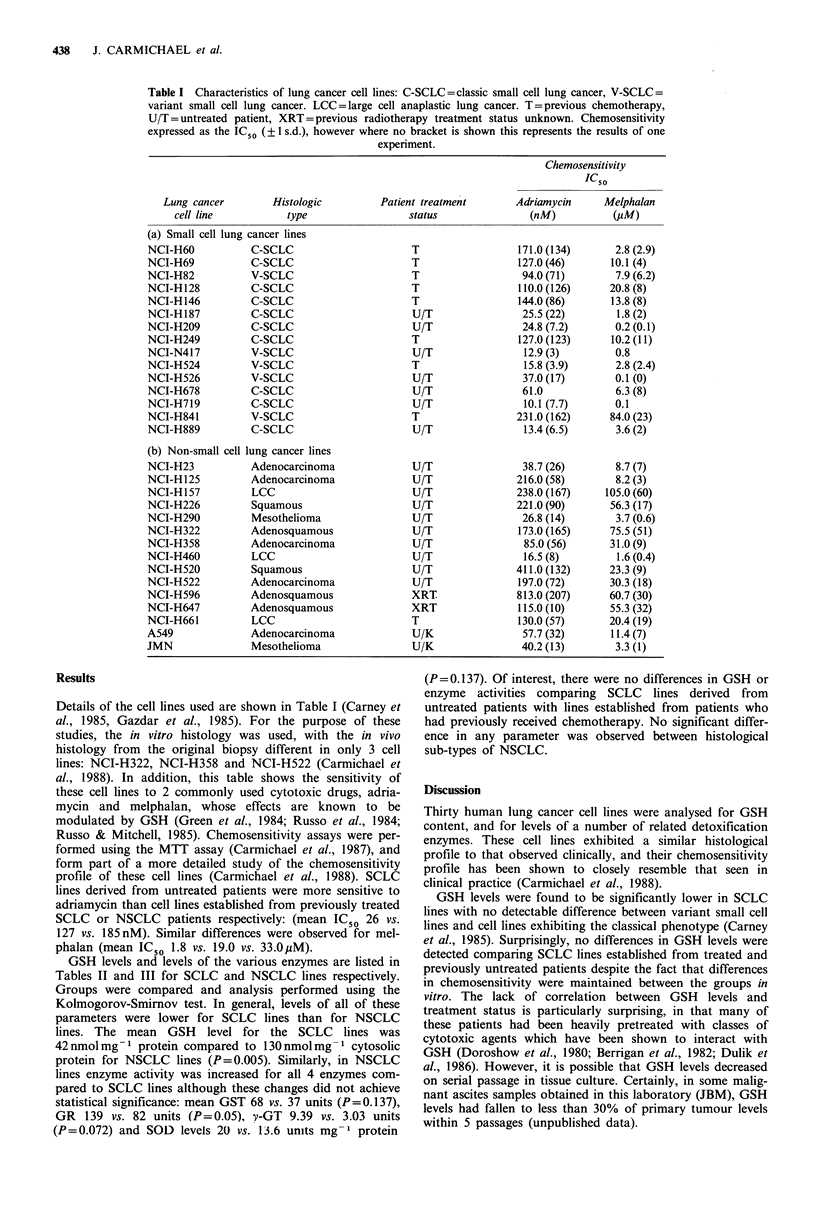
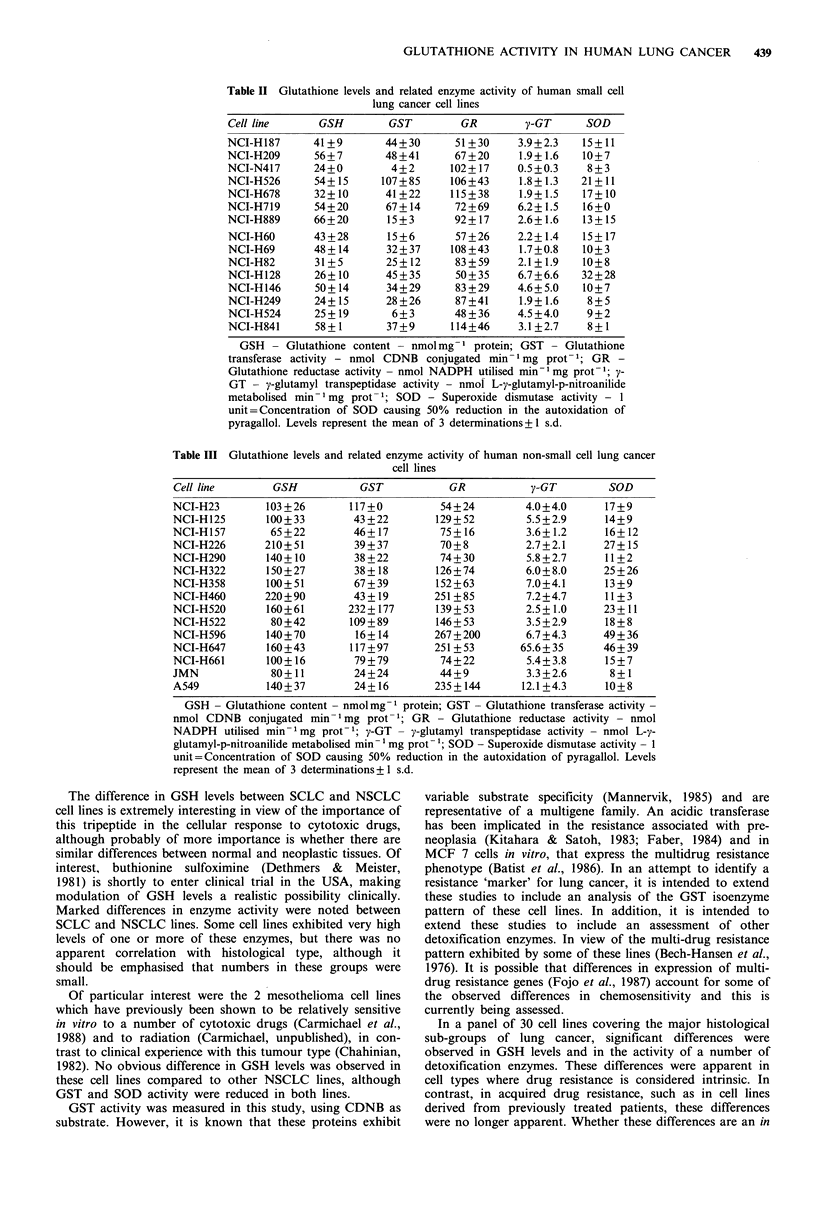
Glutathione levels were measured in 30 human lung cancer lines. Lower levels were detected in cell lines derived from small cell lung cancer specimens compared to non-small cell lines (mean 42 vs. 130 nmol mg-1 protein, P = 0.005). However, no difference were detected between cell lines derived from previously untreated patients, compared to those derived from patients who had received chemotherapy. Non-small cell lines were found to have increased activity of 4 detoxification enzymes compared to small cell lines, although these differences did not reach statistical significance: glutathione transferase activity (69 vs. 36 units, P = 0.137), glutathione reductase (139 vs. 82 units, P = 0.05), gamma-glutamyl transpeptidase (9.39 vs. 3.03 units, P = 0.072) and superoxide dismutase (20 vs. 13.6 units, P = 0.137). As the cell lines exhibit a similar chemosensitivity pattern to that observed in clinical practice, these differences in glutathione and detoxification enzyme levels may prove to be important indicators of intrinsic drug resistance often seen in patients with non-small cell lung cancer.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E., Powrie F., Puri R. N., Meister A. Glutathione monoethyl ester: preparation, uptake by tissues, and conversion to glutathione. Arch Biochem Biophys. 1985 Jun;239(2):538–548. doi: 10.1016/0003-9861(85)90723-4. [DOI] [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Bech-Hansen N. T., Till J. E., Ling V. Pleiotropic phenotype of colchicine-resistant CHO cells: cross-resistance and collateral sensitivity. J Cell Physiol. 1976 May;88(1):23–31. doi: 10.1002/jcp.1040880104. [DOI] [PubMed] [Google Scholar]
- Bergsagel D. E., Feld R. Staging and the evaluation of prognosis for patients with small-cell carcinoma of the lung. J Clin Oncol. 1986 Sep;4(9):1291–1292. doi: 10.1200/JCO.1986.4.9.1291. [DOI] [PubMed] [Google Scholar]
- Berrigan M. J., Marinello A. J., Pavelic Z., Williams C. J., Struck R. F., Gurtoo H. L. Protective role of thiols in cyclophosphamide-induced urotoxicity and depression of hepatic drug metabolism. Cancer Res. 1982 Sep;42(9):3688–3695. [PubMed] [Google Scholar]
- Biaglow J. E., Clark E. P., Epp E. R., Morse-Guadio M., Varnes M. E., Mitchell J. B. Nonprotein thiols and the radiation response of A549 human lung carcinoma cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1983 Nov;44(5):489–495. doi: 10.1080/09553008314551491. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Carmichael J., Mitchell J. B., DeGraff W. G., Gamson J., Gazdar A. F., Johnson B. E., Glatstein E., Minna J. D. Chemosensitivity testing of human lung cancer cell lines using the MTT assay. Br J Cancer. 1988 Jun;57(6):540–547. doi: 10.1038/bjc.1988.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Dethmers J. K., Meister A. Glutathione export by human lymphoid cells: depletion of glutathione by inhibition of its synthesis decreases export and increases sensitivity to irradiation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7492–7496. doi: 10.1073/pnas.78.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshow J. H., Locker G. Y., Myers C. E. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980 Jan;65(1):128–135. doi: 10.1172/JCI109642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulik D. M., Fenselau C., Hilton J. Characterization of melphalan-glutathione adducts whose formation is catalyzed by glutathione transferases. Biochem Pharmacol. 1986 Oct 1;35(19):3405–3409. doi: 10.1016/0006-2952(86)90444-2. [DOI] [PubMed] [Google Scholar]
- Farber E. The biochemistry of preneoplastic liver: a common metabolic pattern in hepatocyte nodules. Can J Biochem Cell Biol. 1984 Jun;62(6):486–494. doi: 10.1139/o84-066. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdar A. F., Carney D. N., Nau M. M., Minna J. D. Characterization of variant subclasses of cell lines derived from small cell lung cancer having distinctive biochemical, morphological, and growth properties. Cancer Res. 1985 Jun;45(6):2924–2930. [PubMed] [Google Scholar]
- Green J. A., Vistica D. T., Young R. C., Hamilton T. C., Rogan A. M., Ozols R. F. Potentiation of melphalan cytotoxicity in human ovarian cancer cell lines by glutathione depletion. Cancer Res. 1984 Nov;44(11):5427–5431. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Jensen G. L., Meister A. Radioprotection of human lymphoid cells by exogenously supplied glutathione is mediated by gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4714–4717. doi: 10.1073/pnas.80.15.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara A., Satoh K., Sato K. Properties of the increased glutathione S-transferase A form in rat preneoplastic hepatic lesions induced by chemical carcinogens. Biochem Biophys Res Commun. 1983 Apr 15;112(1):20–28. doi: 10.1016/0006-291x(83)91791-6. [DOI] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974 Sep 16;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr On the reaction mechanism of yeast glutathione reductase. J Biol Chem. 1965 Nov;240(11):4470–4480. [PubMed] [Google Scholar]
- Mitchell J. B., Russo A., Biaglow J. E., McPherson S. Cellular glutathione depletion by diethyl maleate or buthionine sulfoximine: no effect of glutathione depletion on the oxygen enhancement ratio. Radiat Res. 1983 Nov;96(2):422–428. [PubMed] [Google Scholar]
- Russo A., Mitchell J. B., McPherson S., Friedman N. Alteration of bleomycin cytotoxicity by glutathione depletion or elevation. Int J Radiat Oncol Biol Phys. 1984 Sep;10(9):1675–1678. doi: 10.1016/0360-3016(84)90526-1. [DOI] [PubMed] [Google Scholar]
- Russo A., Mitchell J. B. Potentiation and protection of doxorubicin cytotoxicity by cellular glutathione modulation. Cancer Treat Rep. 1985 Nov;69(11):1293–1296. [PubMed] [Google Scholar]
- Russo A., Mitchell J. B. Radiation response of Chinese hamster cells after elevation of intracellular glutathione levels. Int J Radiat Oncol Biol Phys. 1984 Aug;10(8):1243–1247. doi: 10.1016/0360-3016(84)90326-2. [DOI] [PubMed] [Google Scholar]
- Szasz G. A kinetic photometric method for serum gamma-glutamyl transpeptidase. Clin Chem. 1969 Feb;15(2):124–136. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Williamson J. M., Boettcher B., Meister A. Intracellular cysteine delivery system that protects against toxicity by promoting glutathione synthesis. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6246–6249. doi: 10.1073/pnas.79.20.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]


