Abstract
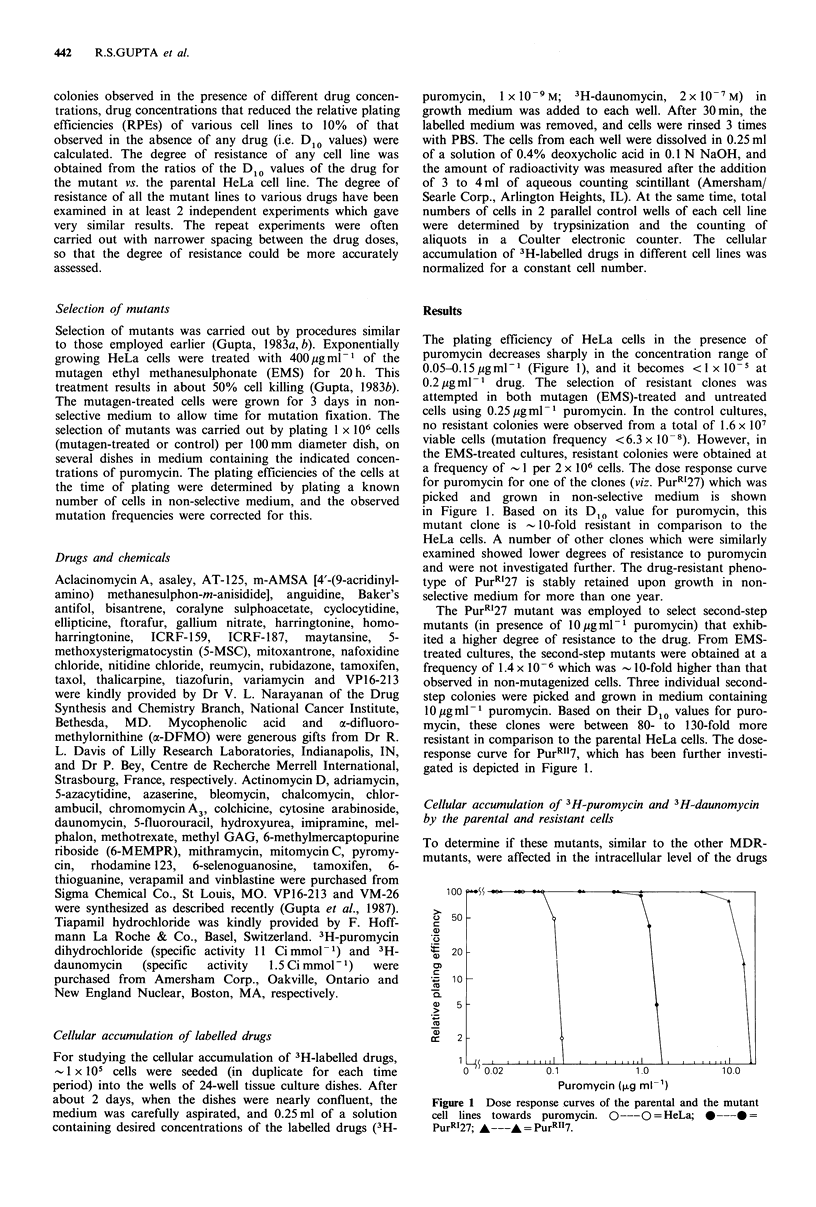
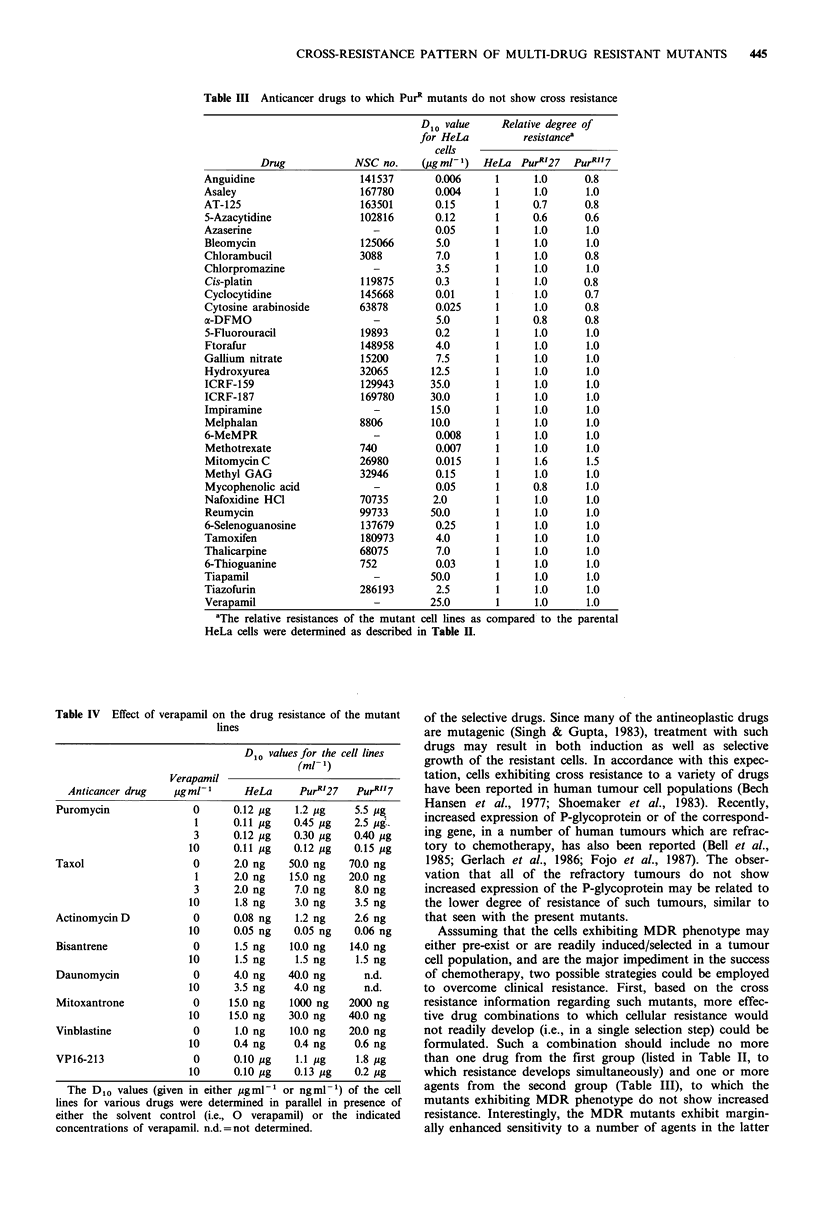
Puromycin-resistant (PurR) mutants/variants of a human carcinoma cell line (HeLa), which show greatly reduced cellular uptake of 3H-puromycin and 3H-daunomycin have been isolated after one- and two-step selections in presence of the drug. The cross-resistance pattern of these mutant cell lines towards numerous anticancer drugs and other inhibitors has been examined. Both the first- and the second-step mutants exhibited increased resistance to a number of antimitotic drugs (viz. vinblastine, vincristine, colchicine, taxol and maytansine), several protein synthesis inhibitors (viz. chalcomycin, bruceantin, harringtonine, homoharringtonine), a large number of DNA interactive compounds (viz. aclacinomycin A, actinomycin D, adriamycin, m-AMSA, chromomycin A3, coralyne sulphoacetate, daunomycin, ellipticine, mithramycin, mitoxantrone, 5-methoxysterigmatocystin, rubidazone, variamycin, VM26 and VP16-213) and a number of other drugs acting via other mechanisms (viz. Baker's antifol, nitidine chloride and rhodamine 123). Whereas the first-step mutants showed stable resistance to these drugs, the second-step lines partially reverted upon growth in non-selective medium. Further, treatment of these mutant lines with non-cytotoxic doses of the calcium channel blocker verapamil reverted or abolished their resistance to the above drugs in a dose-dependent manner. In contrast to the above compounds, the PurR mutants showed no significant cross-resistance to a large number of other drugs which included asaley, AT-125, 5-azacytidine, azaserine, cyclocytidine, cis-platin, cytosine arabinoside, chlorambucil, chlorpromazine, alpha-difluoromethyl ornithine, 5-fluorouracil, ftorafur, gallium nitrate, hydroxyurea, ICRF-159, ICRF-187, imipramine, methotraxate, 6-methylmercaptopurine riboside, mycophenolic acid, melphalan, mitomycin C, methyl GAG, nafoxidine, reumycin, 6-selenoguanosine, 6-thioguanine, tiazofurin, tamoxifen, thalicarpine, tiapamil and verapamil). These cross-resistance data should prove useful in developing suitable drug combinations to which cellular resistance would not develop readily.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Fojo A., Hanover J. A., Pastan I., Gottesman M. M. Isolation and genetic characterization of human KB cell lines resistant to multiple drugs. Somat Cell Mol Genet. 1985 Mar;11(2):117–126. doi: 10.1007/BF01534700. [DOI] [PubMed] [Google Scholar]
- Beck W. T. Cellular pharmacology of Vinca alkaloid resistance and its circumvention. Adv Enzyme Regul. 1984;22:207–227. doi: 10.1016/0065-2571(84)90015-3. [DOI] [PubMed] [Google Scholar]
- Beck W. T. The cell biology of multiple drug resistance. Biochem Pharmacol. 1987 Sep 15;36(18):2879–2887. doi: 10.1016/0006-2952(87)90198-5. [DOI] [PubMed] [Google Scholar]
- Beck W. T. Vinca alkaloid-resistant phenotype in cultured human leukemic lymphoblasts. Cancer Treat Rep. 1983 Oct;67(10):875–882. [PubMed] [Google Scholar]
- Bell D. R., Gerlach J. H., Kartner N., Buick R. N., Ling V. Detection of P-glycoprotein in ovarian cancer: a molecular marker associated with multidrug resistance. J Clin Oncol. 1985 Mar;3(3):311–315. doi: 10.1200/JCO.1985.3.3.311. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Chang T. D., Meyers M. B., Peterson R. H., Spengler B. A. Drug resistance in Chinese hamster lung and mouse tumor cells. Cancer Treat Rep. 1983 Oct;67(10):859–867. [PubMed] [Google Scholar]
- Cass C. E. Density-dependent resistance to puromycin in cell cultures. J Cell Physiol. 1972 Feb;79(1):139–146. doi: 10.1002/jcp.1040790116. [DOI] [PubMed] [Google Scholar]
- Cremisi C., Sonenshein G. E., Tournier P. Studies on the mechanism of actinomycin D resistance of an SV40-transformed hamster cell line. Exp Cell Res. 1974 Nov;89(1):89–94. doi: 10.1016/0014-4827(74)90190-6. [DOI] [PubMed] [Google Scholar]
- Dano K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim Biophys Acta. 1973 Oct 25;323(3):466–483. doi: 10.1016/0005-2736(73)90191-0. [DOI] [PubMed] [Google Scholar]
- Dano K. Cross resistance between vinca alkaloids and anthracyclines in Ehrlich ascites tumor in vivo. Cancer Chemother Rep. 1972 Dec;56(6):701–708. [PubMed] [Google Scholar]
- DeVita V. T., Schein P. S. The use of drugs in combination for the treatment of cancer: rationale and results. N Engl J Med. 1973 May 10;288(19):998–1006. doi: 10.1056/NEJM197305102881905. [DOI] [PubMed] [Google Scholar]
- Ferro-Luzzi Ames G. The basis of multidrug resistance in mammalian cells: homology with bacterial transport. Cell. 1986 Nov 7;47(3):323–324. doi: 10.1016/0092-8674(86)90585-4. [DOI] [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathi R., Grabowski D. Enhancement of sensitivity to adriamycin in resistant P388 leukemia by the calmodulin inhibitor trifluoperazine. Cancer Res. 1983 Aug;43(8):3696–3699. [PubMed] [Google Scholar]
- Goldie J. H., Coldman A. J., Gudauskas G. A. Rationale for the use of alternating non-cross-resistant chemotherapy. Cancer Treat Rep. 1982 Mar;66(3):439–449. [PubMed] [Google Scholar]
- Goldie J. H., Coldman A. J. The genetic origin of drug resistance in neoplasms: implications for systemic therapy. Cancer Res. 1984 Sep;44(9):3643–3653. [PubMed] [Google Scholar]
- Gros P., Ben Neriah Y. B., Croop J. M., Housman D. E. Isolation and expression of a complementary DNA that confers multidrug resistance. Nature. 1986 Oct 23;323(6090):728–731. doi: 10.1038/323728a0. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Chenchaiah P. C., Gupta R. Synthesis and structure-activity relationships among glycosidic derivatives of 4'-demethylepipodophyllotoxin and epipodophyllotoxin, showing VM26- and VP16-213-like activities. Anticancer Drug Des. 1987 Aug;2(1):1–12. [PubMed] [Google Scholar]
- Gupta R. S. Cross-resistance of vinblastine- and taxol-resistant mutants of Chinese hamster ovary cells to other anticancer drugs. Cancer Treat Rep. 1985 May;69(5):515–521. [PubMed] [Google Scholar]
- Gupta R. S. Genetic, biochemical, and cross-resistance studies with mutants of Chinese hamster ovary cells resistant to the anticancer drugs, VM-26 and VP16-213. Cancer Res. 1983 Apr;43(4):1568–1574. [PubMed] [Google Scholar]
- Gupta R. S. Podophyllotoxin-resistant mutants of Chinese hamster ovary cells: cross-resistance studies with various microtubule inhibitors and podophyllotoxin analogues. Cancer Res. 1983 Feb;43(2):505–512. [PubMed] [Google Scholar]
- Inaba M., Fujikura R., Tsukagoshi S., Sakurai Y. Restored in vitro sensitivity of adriamycin- and vincristine-resistant P388 leukemia with reserpine. Biochem Pharmacol. 1981 Aug 1;30(15):2191–2194. doi: 10.1016/0006-2952(81)90246-x. [DOI] [PubMed] [Google Scholar]
- Inaba M., Johnson R. K. Decreased retention of actinomycin D as the basis for cross-resistance in anthracycline-resistant sublines of P388 leukemia. Cancer Res. 1977 Dec;37(12):4629–4634. [PubMed] [Google Scholar]
- Kessel D., Wilberding C. Anthracycline resistance in P388 murine leukemia and its circumvention by calcium antagonists. Cancer Res. 1985 Apr;45(4):1687–1691. [PubMed] [Google Scholar]
- Lieberman I., Ove P. ISOLATION AND STUDY OF MUTANTS FROM MAMMALIAN CELLS IN CULTURE. Proc Natl Acad Sci U S A. 1959 Jun;45(6):867–872. doi: 10.1073/pnas.45.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling V., Kartner N., Sudo T., Siminovitch L., Riordan J. R. Multidrug-resistance phenotype in Chinese hamster ovary cells. Cancer Treat Rep. 1983 Oct;67(10):869–874. [PubMed] [Google Scholar]
- Louie K. G., Hamilton T. C., Winker M. A., Behrens B. C., Tsuruo T., Klecker R. W., Jr, McKoy W. M., Grotzinger K. R., Myers C. E., Young R. C. Adriamycin accumulation and metabolism in adriamycin-sensitive and -resistant human ovarian cancer cell lines. Biochem Pharmacol. 1986 Feb 1;35(3):467–472. doi: 10.1016/0006-2952(86)90221-2. [DOI] [PubMed] [Google Scholar]
- Meyers M. B., Spengler B. A., Chang T. D., Melera P. W., Biedler J. L. Gene amplification-associated cytogenetic aberrations and protein changes in vincristine-resistant Chinese hamster, mouse, and human cells. J Cell Biol. 1985 Feb;100(2):588–597. doi: 10.1083/jcb.100.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J., Sammons D., Barron E. Puromycin resistance in Chinese hamster cells: genetic and biochemical studies of partially resistant, unstable clones. Mutat Res. 1980 Feb;69(2):333–346. doi: 10.1016/0027-5107(80)90098-6. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Deuchars K., Kartner N., Alon N., Trent J., Ling V. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. 1985 Aug 29-Sep 4Nature. 316(6031):817–819. doi: 10.1038/316817a0. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Genetic and biochemical characterization of multidrug resistance. Pharmacol Ther. 1985;28(1):51–75. doi: 10.1016/0163-7258(85)90082-8. [DOI] [PubMed] [Google Scholar]
- Rogan A. M., Hamilton T. C., Young R. C., Klecker R. W., Jr, Ozols R. F. Reversal of adriamycin resistance by verapamil in human ovarian cancer. Science. 1984 Jun 1;224(4652):994–996. doi: 10.1126/science.6372095. [DOI] [PubMed] [Google Scholar]
- Roninson I. B., Abelson H. T., Housman D. E., Howell N., Varshavsky A. Amplification of specific DNA sequences correlates with multi-drug resistance in Chinese hamster cells. Nature. 1984 Jun 14;309(5969):626–628. doi: 10.1038/309626a0. [DOI] [PubMed] [Google Scholar]
- Schabel F. M., Jr, Skipper H. E., Trader M. W., Laster W. R., Jr, Griswold D. P., Jr, Corbett T. H. Establishment of cross-resistance profiles for new agents. Cancer Treat Rep. 1983 Oct;67(10):905–922. [PubMed] [Google Scholar]
- Shoemaker R. H., Curt G. A., Carney D. N. Evidence for multidrug-resistant cells in human tumor cell populations. Cancer Treat Rep. 1983 Oct;67(10):883–888. [PubMed] [Google Scholar]
- Singh B., Gupta R. S. Mutagenic responses of thirteen anticancer drugs on mutation induction at multiple genetic loci and on sister chromatid exchanges in Chinese hamster ovary cells. Cancer Res. 1983 Feb;43(2):577–584. [PubMed] [Google Scholar]
- Singh B., Gupta R. S. Species-specific differences in the toxicity and mutagenicity of the anticancer drugs mithramycin, chromomycin A3, and olivomycin. Cancer Res. 1985 Jun;45(6):2813–2820. [PubMed] [Google Scholar]
- Skovsgaard T. Mechanism of cross-resistance between vincristine and daunorubicin in Ehrlich ascites tumor cells. Cancer Res. 1978 Dec;38(12):4722–4727. [PubMed] [Google Scholar]
- Slater L. M., Murray S. L., Wetzel M. W., Wisdom R. M., DuVall E. M. Verapamil restoration of daunorubicin responsiveness in daunorubicin-resistant Ehrlich ascites carcinoma. J Clin Invest. 1982 Nov;70(5):1131–1134. doi: 10.1172/JCI110702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Kitatani Y., Yokota K., Tsukagoshi S., Sakurai Y. Effects of quinidine and related compounds on cytotoxicity and cellular accumulation of vincristine and adriamycin in drug-resistant tumor cells. Cancer Res. 1984 Oct;44(10):4303–4307. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981 May;41(5):1967–1972. [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., Bleehen N. M. Drug resistance in human lung cancer cell lines: cross-resistance studies and effects of the calcium transport blocker, verapamil. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1355–1358. doi: 10.1016/0360-3016(86)90170-7. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., Wright K. A., Bleehen N. M. Derivation and preliminary characterisation of adriamycin resistant lines of human lung cancer cells. Br J Cancer. 1986 Apr;53(4):529–537. doi: 10.1038/bjc.1986.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr J. R., Brewer F., Anderson M., Fergusson J. Verapamil hypersensitivity of vincristine resistant Chinese hamster ovary cell lines. Cell Biol Int Rep. 1986 May;10(5):389–399. doi: 10.1016/0309-1651(86)90011-1. [DOI] [PubMed] [Google Scholar]


