Abstract
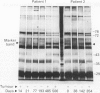
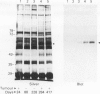
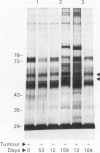
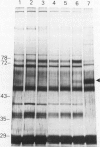
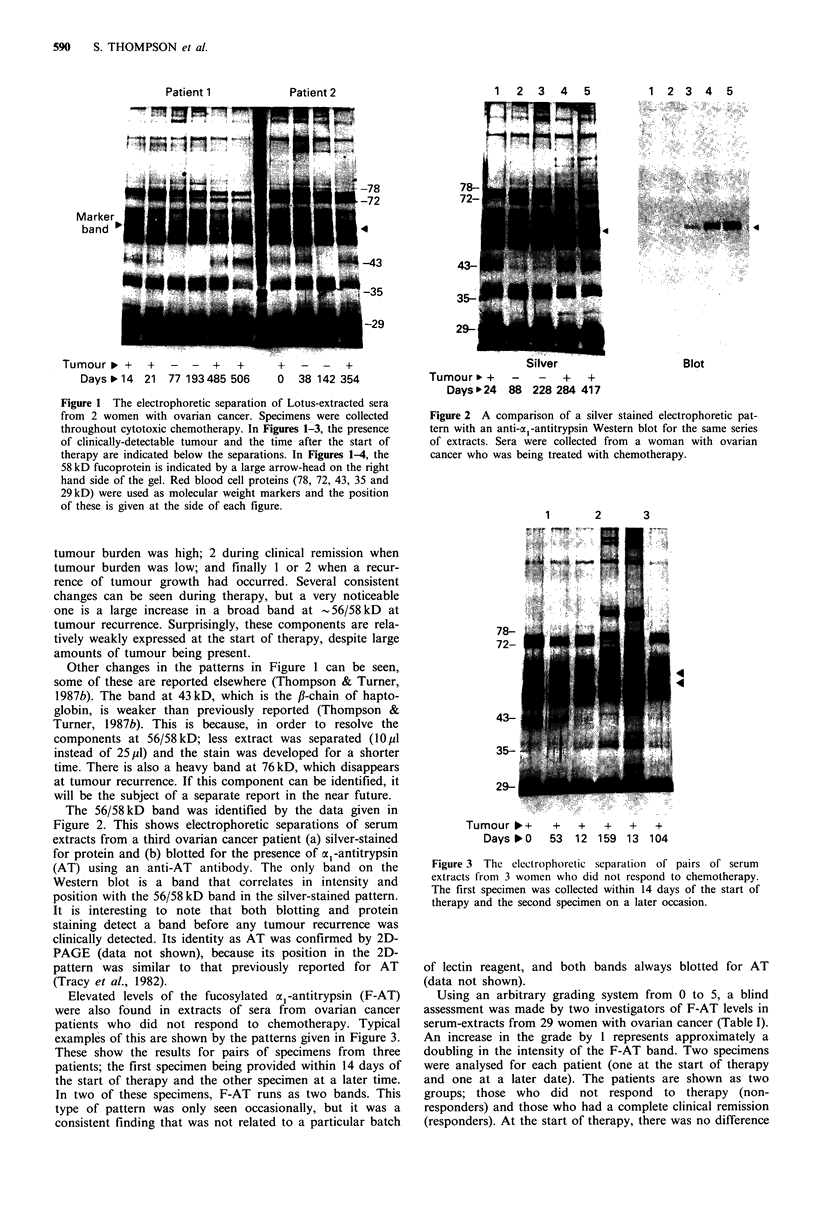
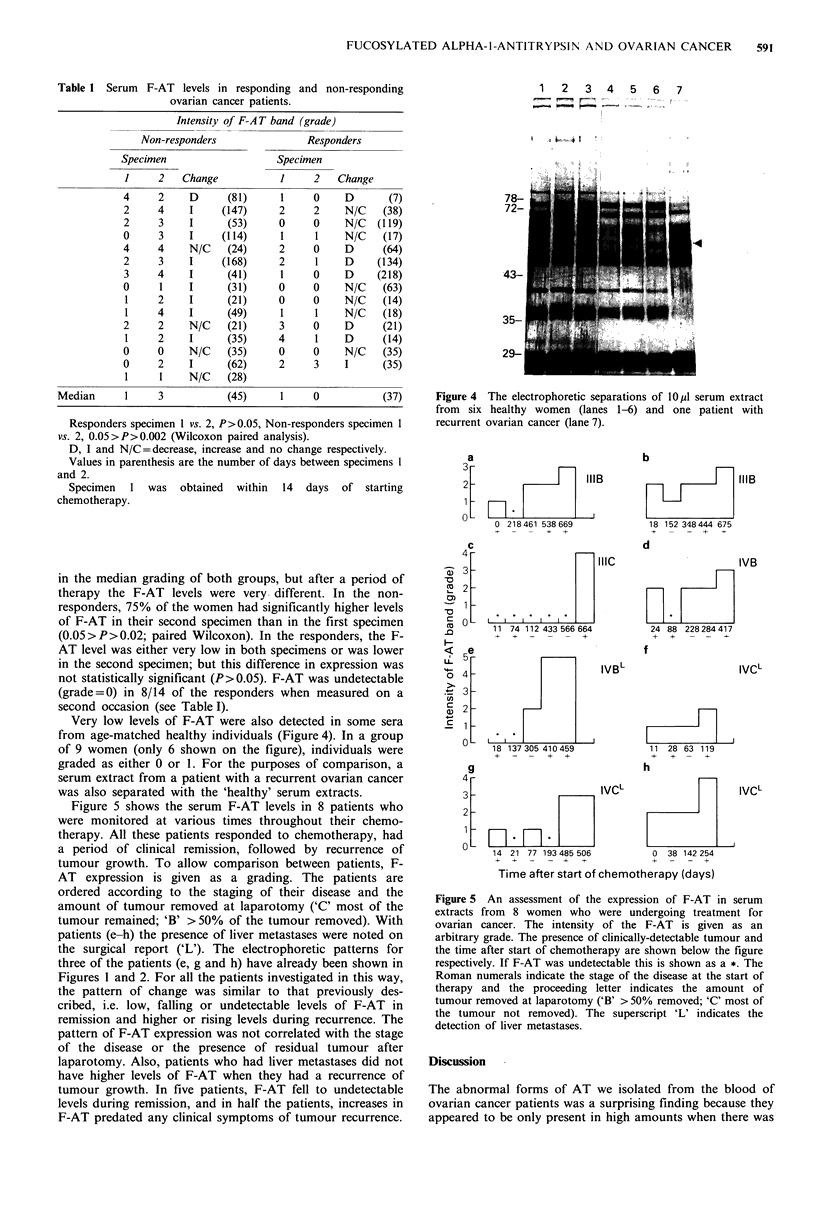
We have discovered modified fucosylation of alpha 1-antitrypsin (F-AT) in the sera of ovarian cancer patients. This was detected by SDS/electrophoresis and silver-staining after extracting the sera with the fucose-binding lectin, Lotus tetragonolobus, and was identified as alpha 1-antitrypsin by Western blotting. Initially, high F-AT levels appeared to be related to the recurrence of cancer, but later measurements showed that elevated levels were also present in patients who did not respond to therapy. Using an arbitrary grading system, the level of F-AT was assessed in pairs of sera from 29 ovarian cancer patients undergoing therapy; one specimen collected just after the start of therapy and the other on a later occasion. In 75% of the 15 non-responders, F-AT was higher when measured on a second occasion; whereas in 86% of the 14 responders the second measurement was either unchanged or lower, being frequently undetectable. F-AT levels were also low or undetectable in sera from healthy women. Eight responders were monitored for F-AT throughout cyclophosphamide chemotherapy. Despite a high tumour burden at the start of therapy, all patients had relatively low levels of F-AT and this was maintained throughout remission; the levels only becoming elevated with the recurrence of tumour growth. Increased F-AT expression did not appear to be particularly associated with the presence of liver metastases and frequently predated any clinical signs of a recurrence. The interesting characteristics of these molecules could make them useful in the management of ovarian cancer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayard B., Kerckaert J. P., Laine A., Hayem A. Uniformity of glycans within molecular variants of alpha-protease inhibitor with distinct affinity for concanavalin A. Eur J Biochem. 1982 May 17;124(2):371–376. doi: 10.1111/j.1432-1033.1982.tb06602.x. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Carlson J., Eriksson S., Alm R., Kjellström T. Biosynthesis of abnormally glycosylated alpha 1-antitrypsin by a human hepatoma cell line. Hepatology. 1984 Mar-Apr;4(2):235–241. doi: 10.1002/hep.1840040211. [DOI] [PubMed] [Google Scholar]
- Chan S. K., Luby J., Wu Y. C. Purification and chemical compositions of human alpha1-antitrypsin of the MM type. FEBS Lett. 1973 Sep 1;35(1):79–82. doi: 10.1016/0014-5793(73)80581-2. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Purification and properties of normal human alpha 1-antitrypsin. Arch Biochem Biophys. 1973 May;156(1):215–222. doi: 10.1016/0003-9861(73)90359-7. [DOI] [PubMed] [Google Scholar]
- Evans A. S., Dolan M. F., Sobocinski P. Z., Quinn F. A. Utility of serum protein-bound neutral hexoses and L-fucose for estimation of malignant tumor extension and evaluation of efficacy of therapy. Cancer Res. 1974 Mar;34(3):538–542. [PubMed] [Google Scholar]
- Guthrie D. The treatment of advanced cystadenocarcinoma of the ovary with gestronol and continuous oral cyclophosphamide. Br J Obstet Gynaecol. 1979 Jul;86(7):497–500. doi: 10.1111/j.1471-0528.1979.tb10799.x. [DOI] [PubMed] [Google Scholar]
- HEIMBURGER N., HEIDE K., HAUPT H., SCHULTZE H. E. BAUSTEINANALYSEN VON HUMANSERUMPROTEINEN. Clin Chim Acta. 1964 Oct;10:293–307. doi: 10.1016/0009-8981(64)90059-2. [DOI] [PubMed] [Google Scholar]
- Hercz A. The oligosaccharides of human alpha 1-antitrypsin. Can J Biochem Cell Biol. 1984 Jan;62(1):19–27. doi: 10.1139/o84-004. [DOI] [PubMed] [Google Scholar]
- Hodges L. C., Chan S. K. Locations of oligosaccharide chains in human alpha 1-protease inhibitor and oligosaccharide structures at each site. Biochemistry. 1982 May 25;21(11):2805–2810. doi: 10.1021/bi00540a036. [DOI] [PubMed] [Google Scholar]
- Laurell C. B., Rannevik G. A comparison of plasma protein changes induced by danazol, pregnancy, and estrogens. J Clin Endocrinol Metab. 1979 Nov;49(5):719–725. doi: 10.1210/jcem-49-5-719. [DOI] [PubMed] [Google Scholar]
- Mega T., Lujan E., Yoshida A. Studies on the oligosaccharide chains of human alpha 1-protease inhibitor. II. Structure of oligosaccharides. J Biol Chem. 1980 May 10;255(9):4057–4061. [PubMed] [Google Scholar]
- Petryniak J., Goldstein I. J. Immunochemical studies on the interaction between synthetic glycoconjugates and alpha-L-fucosyl binding lectins. Biochemistry. 1986 May 20;25(10):2829–2838. doi: 10.1021/bi00358a014. [DOI] [PubMed] [Google Scholar]
- Thompson S., Latham J. A., Turner G. A. A simple, reproducible and cheap batch method for the analysis of serum glycoproteins using sepharose-coupled lectins and silver staining. Clin Chim Acta. 1987 Aug 14;167(2):217–223. doi: 10.1016/0009-8981(87)90374-3. [DOI] [PubMed] [Google Scholar]
- Thompson S., Turner G. A. Elevated levels of abnormally-fucosylated haptoglobins in cancer sera. Br J Cancer. 1987 Nov;56(5):605–610. doi: 10.1038/bjc.1987.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy R. P., Currie R. M., Young D. S. Two-dimensional gel electrophoresis of serum specimens from a normal population. Clin Chem. 1982 Apr;28(4 Pt 2):890–899. [PubMed] [Google Scholar]
- Turner G. A., Ellis R. D., Guthrie D., Latner A. L., Ross W. M., Skillen A. W. Cyclic GMP in urine to monitor the response to ovarian cancer to therapy. Br J Obstet Gynaecol. 1982 Sep;89(9):760–764. doi: 10.1111/j.1471-0528.1982.tb05105.x. [DOI] [PubMed] [Google Scholar]
- Turner G. A., Skillen A. W., Buamah P., Guthrie D., Welsh J., Harrison J., Kowalski A. Relation between raised concentrations of fucose, sialic acid, and acute phase proteins in serum from patients with cancer: choosing suitable serum glycoprotein markers. J Clin Pathol. 1985 May;38(5):588–592. doi: 10.1136/jcp.38.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes T. P., Mrochek J. E., Dinsmore S. R., Tormey D. C. Serum protein-bound carbohydrates for following the course of disease in patients with metastatic breast carcinoma. J Natl Cancer Inst. 1978 Sep;61(3):703–707. [PubMed] [Google Scholar]