Abstract
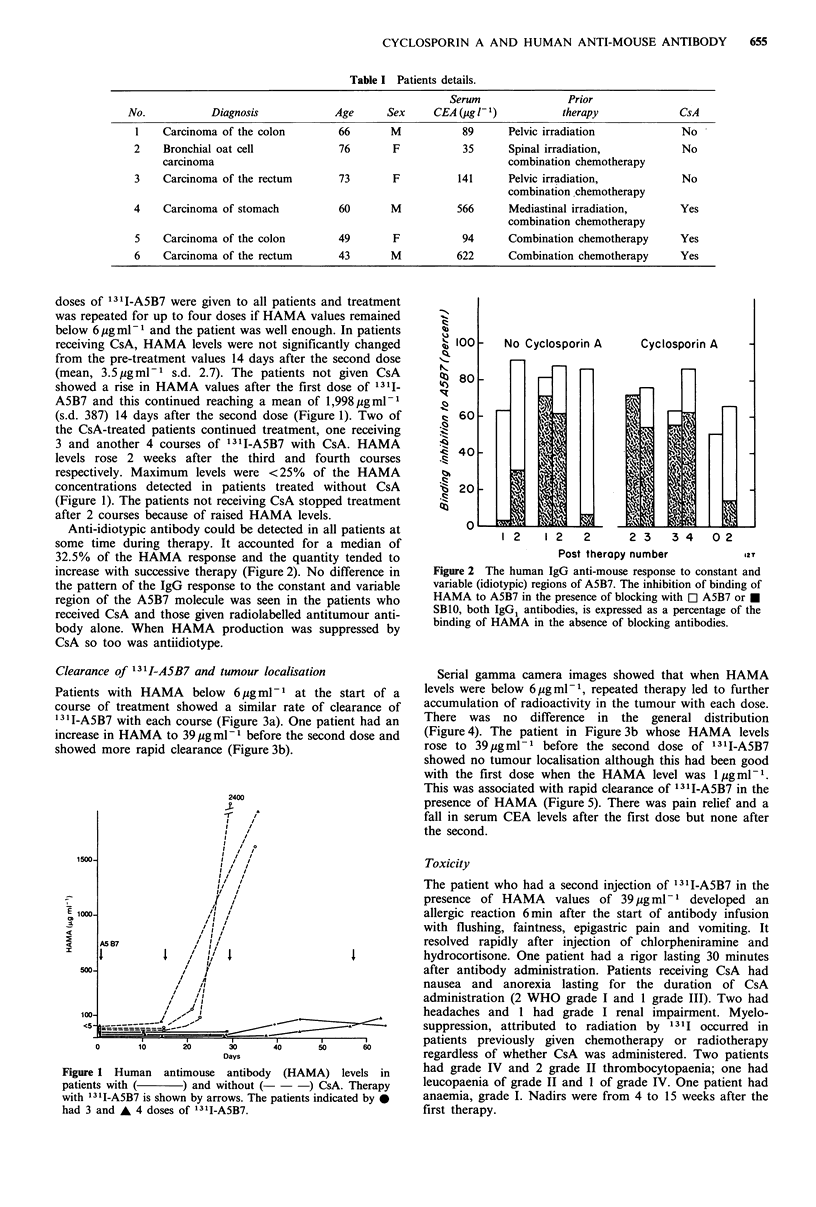
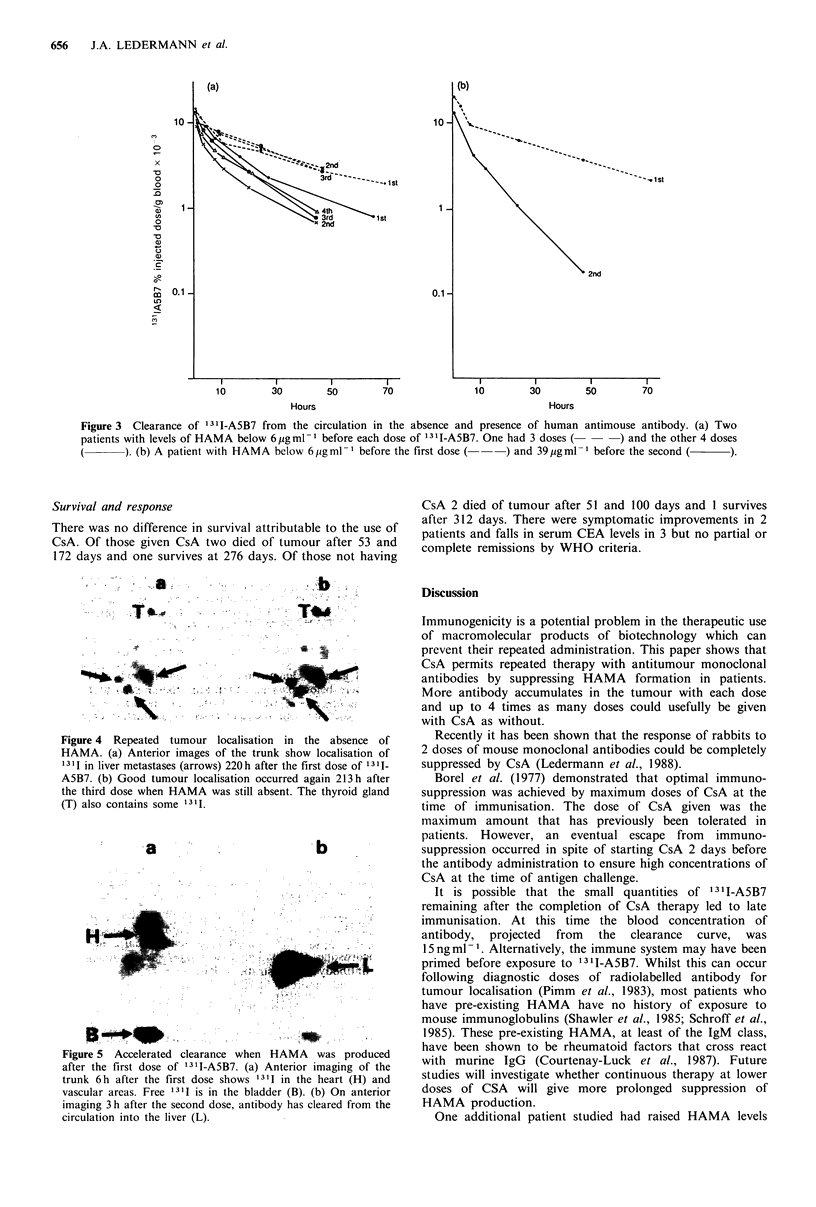
Antibody targeted therapy of cancer results in anti-antibody production which prevents repeated treatment. Cyclosporin A (CsA) has been used to suppress this response in patients treated with a radiolabelled antibody to carcinoembryonic antigen (CEA). Patients with CEA producing tumours received a minimum of two courses consisting of an injection of radiolabelled antibody and CsA, 24 mg kg-1 day-1, for 6 days; each course was given at 2 week intervals. Two weeks after the completion of the second course the mean human antimouse antibody (HAMA) levels were 3.5 micrograms ml-1 (s.d. 2.7) in 3 patients receiving CsA and 1,998 micrograms ml-1 (s.d. 387) in 3 patients not receiving the drug. Clearance of antitumour antibody was accelerated and tumour localisation absent when HAMA levels exceeded 30 micrograms ml-1. With lower levels of HAMA in the CsA-treated patients, further antitumour antibody accumulated in the tumour after each dose. Further therapy with antitumour antibody and CsA lead to the development of HAMA, but this was less than 25% of the amount in patients not given CsA. In this preliminary study up to 4 times as many doses of antitumour antibody could be usefully given when CsA was used. This increases the potential for effective antibody targeted therapy of cancer.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshawe K. D. Antibody directed enzymes revive anti-cancer prodrugs concept. Br J Cancer. 1987 Nov;56(5):531–532. doi: 10.1038/bjc.1987.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Gubler H. U., Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976 Jul;6(4):468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo J. A., Krohn K. A., Beaumier P., McGuffin R. W., Brown J. P., Hellström K. E., Hellström I., Larson S. M. Diagnosis of and therapy for solid tumors with radiolabeled antibodies and immune fragments. Cancer Treat Rep. 1984 Jan;68(1):317–328. [PubMed] [Google Scholar]
- Courtenay-Luck N. S., Epenetos A. A., Winearls C. G., Ritter M. A. Preexisting human anti-murine immunoglobulin reactivity due to polyclonal rheumatoid factors. Cancer Res. 1987 Aug 15;47(16):4520–4525. [PubMed] [Google Scholar]
- Harwood P. J., Britton D. W., Southall P. J., Boxer G. M., Rawlins G., Rogers G. T. Mapping epitope characteristics on carcinoembryonic antigen. Br J Cancer. 1986 Jul;54(1):75–82. doi: 10.1038/bjc.1986.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffers G. J., Fuller T. C., Cosimi A. B., Russell P. S., Winn H. J., Colvin R. B. Monoclonal antibody therapy. Anti-idiotypic and non-anti-idiotypic antibodies to OKT3 arising despite intense immunosuppression. Transplantation. 1986 May;41(5):572–578. doi: 10.1097/00007890-198605000-00004. [DOI] [PubMed] [Google Scholar]
- Ledermann J. A., Begent R. H., Bagshawe K. D. Cyclosporin A prevents the anti-murine antibody response to a monoclonal anti-tumour antibody in rabbits. Br J Cancer. 1988 Nov;58(5):562–566. doi: 10.1038/bjc.1988.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard R. E., Jr, Order S. E., Spunberg J. J., Asbell S. O., Leibel S. A. Isotopic immunoglobulin: a new systemic therapy for advanced Hodgkin's disease. J Clin Oncol. 1985 Oct;3(10):1296–1300. doi: 10.1200/JCO.1985.3.10.1296. [DOI] [PubMed] [Google Scholar]
- Lindsey N. J., Harris K. R., Norman H. B., Smith J. L., Lee H. A., Slapek M. The effect of cyclosporin A on the primary and secondary immune responses in the rabbit. Transplant Proc. 1980 Jun;12(2):252–255. [PubMed] [Google Scholar]
- Meeker T. C., Lowder J., Maloney D. G., Miller R. A., Thielemans K., Warnke R., Levy R. A clinical trial of anti-idiotype therapy for B cell malignancy. Blood. 1985 Jun;65(6):1349–1363. [PubMed] [Google Scholar]
- Order S. E., Stillwagon G. B., Klein J. L., Leichner P. K., Siegelman S. S., Fishman E. K., Ettinger D. S., Haulk T., Kopher K., Finney K. Iodine 131 antiferritin, a new treatment modality in hepatoma: a Radiation Therapy Oncology Group study. J Clin Oncol. 1985 Dec;3(12):1573–1582. doi: 10.1200/JCO.1985.3.12.1573. [DOI] [PubMed] [Google Scholar]
- Pectasides D., Stewart S., Courtenay-Luck N., Rampling R., Munro A. J., Krausz T., Dhokia B., Snook D., Hooker G., Durbin H. Antibody-guided irradiation of malignant pleural and pericardial effusions. Br J Cancer. 1986 Jun;53(6):727–732. doi: 10.1038/bjc.1986.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley R. B., Boden J., Keep P. A., Harwood P. J., Green A. J., Rogers G. T. Relationship between tumour size and uptake of radiolabelled anti-CEA in a colon tumour xenograft. Eur J Nucl Med. 1987;13(4):197–202. doi: 10.1007/BF00256491. [DOI] [PubMed] [Google Scholar]
- Pimm M. V., Perkins A. C., Armitage N. C., Baldwin R. W. The characteristics of blood-borne radiolabels and the effect of anti-mouse IgG antibodies on localization of radiolabeled monoclonal antibody in cancer patients. J Nucl Med. 1985 Sep;26(9):1011–1023. [PubMed] [Google Scholar]
- Raso V. Antibody mediated delivery of toxic molecules to antigen bearing target cells. Immunol Rev. 1982;62:93–117. doi: 10.1111/j.1600-065x.1982.tb00391.x. [DOI] [PubMed] [Google Scholar]
- Schroff R. W., Foon K. A., Beatty S. M., Oldham R. K., Morgan A. C., Jr Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res. 1985 Feb;45(2):879–885. [PubMed] [Google Scholar]
- Shawler D. L., Bartholomew R. M., Smith L. M., Dillman R. O. Human immune response to multiple injections of murine monoclonal IgG. J Immunol. 1985 Aug;135(2):1530–1535. [PubMed] [Google Scholar]
- Spitler L. E., del Rio M., Khentigan A., Wedel N. I., Brophy N. A., Miller L. L., Harkonen W. S., Rosendorf L. L., Lee H. M., Mischak R. P. Therapy of patients with malignant melanoma using a monoclonal antimelanoma antibody-ricin A chain immunotoxin. Cancer Res. 1987 Mar 15;47(6):1717–1723. [PubMed] [Google Scholar]
- Thistlethwaite J. R., Jr, Cosimi A. B., Delmonico F. L., Rubin R. H., Talkoff-Rubin N., Nelson P. W., Fang L., Russell P. S. Evolving use of OKT3 monoclonal antibody for treatment of renal allograft rejection. Transplantation. 1984 Dec;38(6):695–701. doi: 10.1097/00007890-198412000-00029. [DOI] [PubMed] [Google Scholar]