Abstract
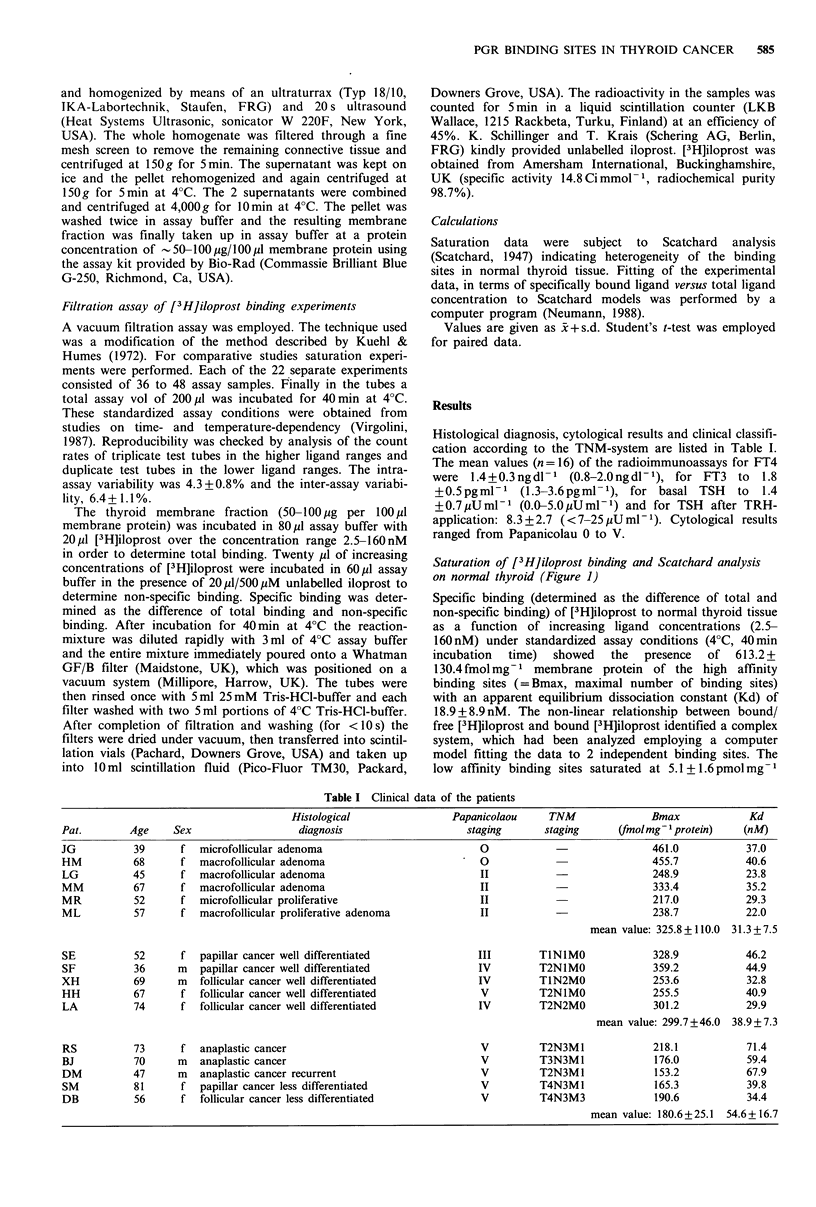
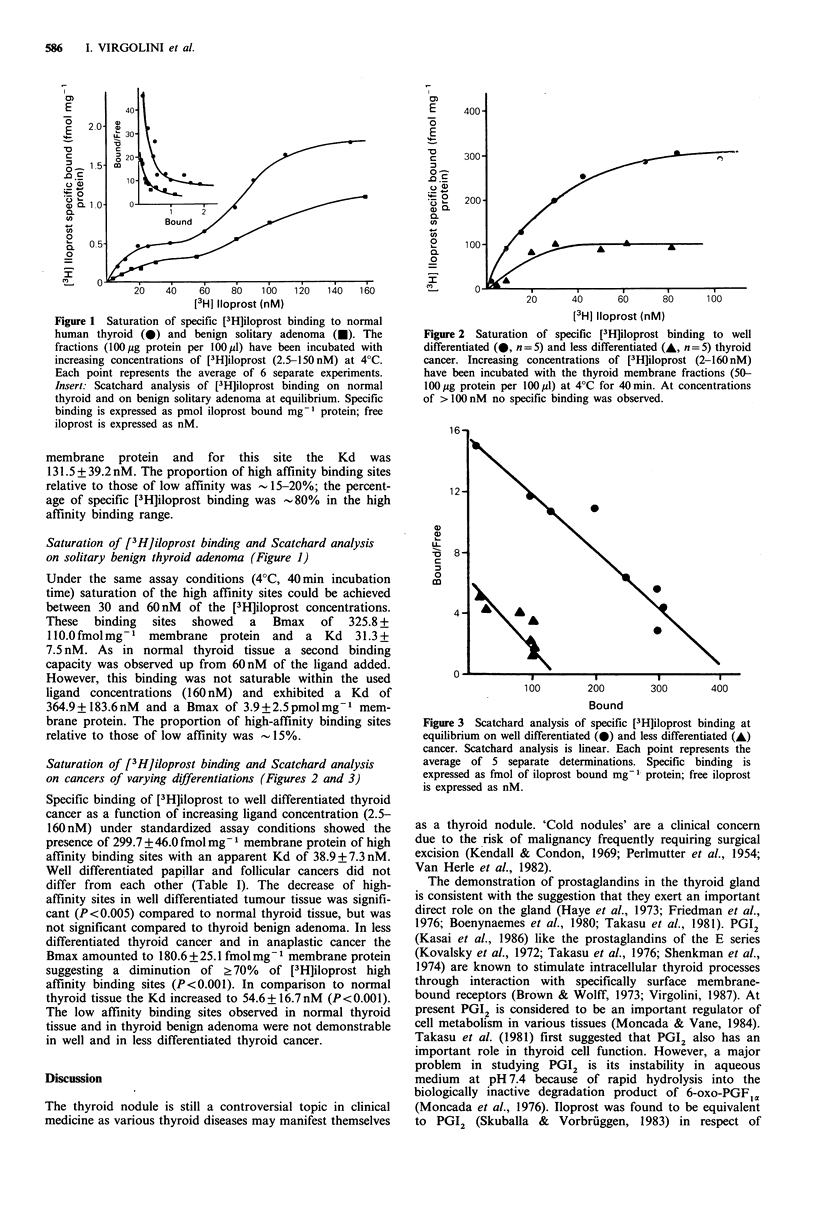
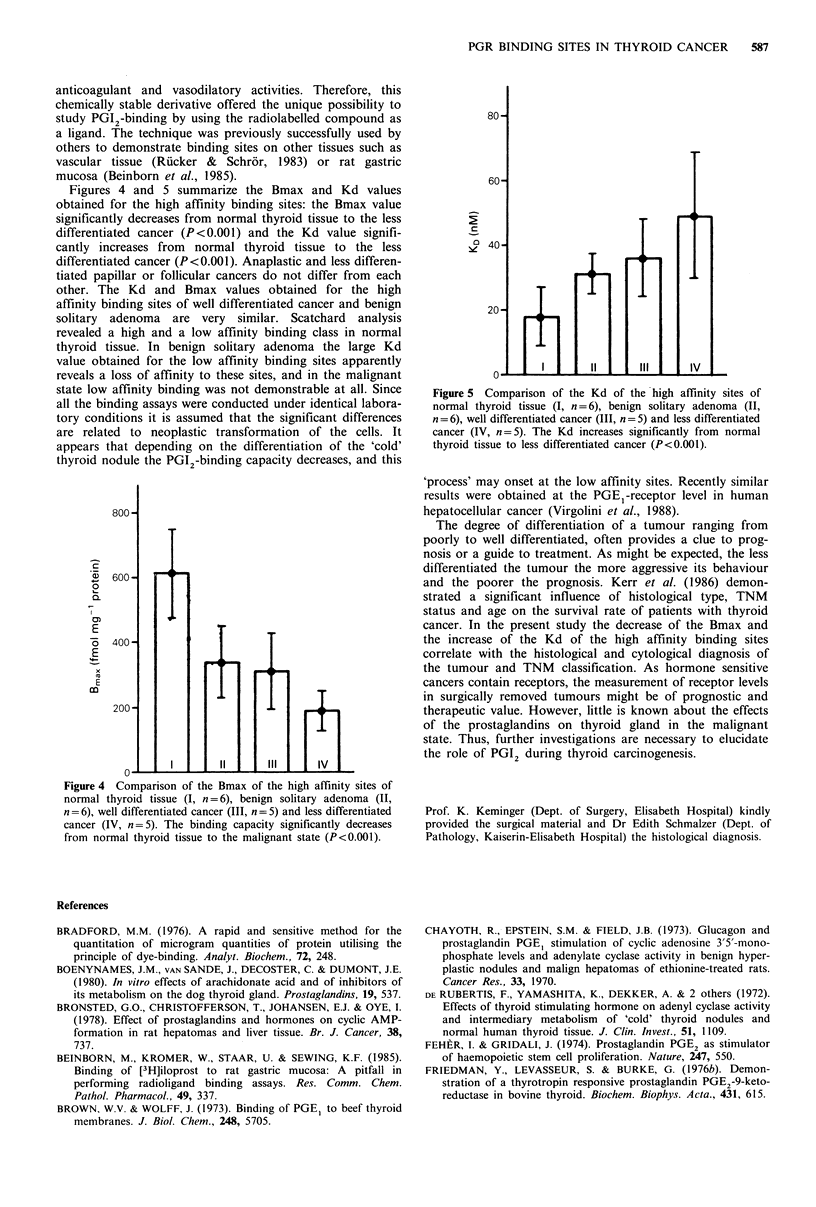
The properties of specific prostaglandin I2 (prostacyclin, PGI2) binding sites in normal thyroid tissue have been characterised. Tissue samples obtained intraoperatively from patients with 'cold' solitary thyroid nodules (as preoperatively selected by thyroid gland scintigraphy, thyroid gland ultrasonography and Papanicolaou cytology following fine needle aspiration of the nodule area) have been used for thyroid membrane preparation. Employing [3H]iloprost, a chemically stable PGI2-analogue as a radioligand, saturation experiments for comparative binding studies have been attempted. Scatchard analysis of the binding data obtained for normal thyroid parenchyma distant to the nodule area revealed heterogeneity of the [3H]iloprost sites exhibiting a high-affinity binding capacity (Bmax) of 613.2 +/- 130.4 fmol mg-1 membrane protein and a low-affinity binding capacity of 5.1 +/- 1.6 pmol mg-1 membrane protein. The equilibrium dissociation constant (Kd) amounted to 18.9 +/- 8.9 nM and to 131.5 +/- 39.2 nM, respectively. Scatchard analysis of the binding data obtained for benign thyroid adenoma indicated significant lower binding capacities exhibiting a Bmax of 325.8 +/- 110.0 fmol mg-1 membrane protein (Kd: 31.0 +/- 7.5 nM) for the high-affinity sites and of 3.9 +/- 2.5 pmol mg-1 membrane protein (Kd: 364.9 +/- 183.6) for the low affinity sites. In cancer tissue a selective loss of the low affinity sites and a significant diminution of the high-affinity sites was observed: in well differentiated cancer the high-affinity sites showed a Bmax of 299.7 +/- 46.0 fmol mg-1 membrane protein (Kd: 38.9 +/- 7.3 nM), in anaplastic cancer, less differentiated papillar and follicular cancers of 180.6 +/- 25.1 fmol mg-1 membrane protein (Kd: 54.6 +/- 16.7 nM). Well differentiated papillar and follicular cancers did not differ from each other.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinborn M., Kromer W., Staar U., Sewing K. F. Binding of 3H-iloprost to rat gastric mucosa: a pitfall in performing radioligand binding assays. Res Commun Chem Pathol Pharmacol. 1985 Sep;49(3):337–351. [PubMed] [Google Scholar]
- Boeynaems J. M., Van Sande J., Decoster C., Dumont J. E. In vitro effects of arachidonic acid and of inhibitors of its metabolism on the dog thyroid gland. Prostaglandins. 1980 Apr;19(4):537–550. doi: 10.1016/s0090-6980(80)80004-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brønstad G. O., Christoffersen T., Johansen E. J., Oye I. Effect of prostaglandins and hormones on cyclic AMP formation in rat hepatomas and liver tissue. Br J Cancer. 1978 Dec;38(6):737–744. doi: 10.1038/bjc.1978.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayoth R., Epstein S. M., Field J. B. Glucagon and prostaglandin E1 stimulation of cyclic adenosine 3', 5'-monophosphate levels and adenylate cyclase activity in benign hyperplastic nodules and malignant hepatomas of ethionine-treated rats. Cancer Res. 1973 Aug;33(8):1970–1974. [PubMed] [Google Scholar]
- DeRubertis F., Yamashita K., Dekker A., Larsen P. R., Field J. B. Effects of thyroid-stimulating hormone on adenyl cyclase activity and intermediary metabolism of "cold" thyroid nodules and normal human thyroid tissue. J Clin Invest. 1972 May;51(5):1109–1117. doi: 10.1172/JCI106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehér I., Gidáli J. Prostaglandin E2 as stimulator of haemopoietic stem cell proliferation. Nature. 1974 Feb 22;247(5442):550–551. doi: 10.1038/247550a0. [DOI] [PubMed] [Google Scholar]
- Friedman Y., Levasseur S., Burke G. Demonstration of a thyrotropin-responsive prostaglandin E2-9-ketoreductase in bovine thyroid. Biochim Biophys Acta. 1976 Jun 22;431(3):615–623. doi: 10.1016/0005-2760(76)90225-3. [DOI] [PubMed] [Google Scholar]
- Hall J. M., Strange P. G. The use of a prostacyclin analogue, [3H]iloprost, for studying prostacyclin-binding sites on human platelets and neuronal hybrid cells. Biosci Rep. 1984 Nov;4(11):941–948. doi: 10.1007/BF01116892. [DOI] [PubMed] [Google Scholar]
- Haye B., Champion S., Jacquemin C. Existence of two pools of prostaglandins during stimulation of the thyroid by TSH. FEBS Lett. 1974 Apr 15;41(1):89–93. doi: 10.1016/0014-5793(74)80961-0. [DOI] [PubMed] [Google Scholar]
- Hial V., Horakova Z., Shaff F. E., Beaven M. A. Alteration of tumor growth by aspirin and indomethacin: studies with two transplantable tumors in mouse. Eur J Pharmacol. 1976 Jun;37(2):367–376. doi: 10.1016/0014-2999(76)90044-3. [DOI] [PubMed] [Google Scholar]
- Kasai K., Hiraiwa M., Suzuki Y., Banba N., Emoto T., Nakamura T., Shimoda S. I. Prostacyclin stimulation of adenylate cyclase activity in human thyroid membranes. Horm Metab Res. 1986 Sep;18(9):625–629. doi: 10.1055/s-2007-1012390. [DOI] [PubMed] [Google Scholar]
- Kendall L. W., Condon R. E. Prediction of malignancy in solitary thyroid nodules. Lancet. 1969 May 31;1(7605):1071–1073. doi: 10.1016/s0140-6736(69)91707-3. [DOI] [PubMed] [Google Scholar]
- Kerr D. J., Burt A. D., Boyle P., MacFarlane G. J., Storer A. M., Brewin T. B. Prognostic factors in thyroid tumours. Br J Cancer. 1986 Sep;54(3):475–482. doi: 10.1038/bjc.1986.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski K., Sato S., Burke G. Thyrotropin- and prostaglandin E2-responsive adenyl cyclase in thyroid plasma membranes. Prostaglandins. 1972 Dec;2(6):441–452. doi: 10.1016/s0090-6980(72)80031-5. [DOI] [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Humes J. L. Direct evidence for a prostaglandin receptor and its application to prostaglandin measurements (rat-adipocytes-antagonists-analogues-mouse ovary assay). Proc Natl Acad Sci U S A. 1972 Feb;69(2):480–484. doi: 10.1073/pnas.69.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Gryglewski R., Bunting S., Vane J. R. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976 Oct 21;263(5579):663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Moore W. V., Wolff J. Binding of prostaglandin E1 to beef thyroid membranes. J Biol Chem. 1973 Aug 25;248(16):5705–5711. [PubMed] [Google Scholar]
- Orgiazzi J., Munari Y., Rostagnat A., Dutrieux N., Mornex R. Adenyl cyclase activity in thyroid carcinomas. Ann Radiol (Paris) 1977 Nov-Dec;20(8):757–759. [PubMed] [Google Scholar]
- PERLMUTTER M., SLATER S. L., ATTIE J. Method for preoperative differentiation between the benign and the possibly malignant solitary nontoxic thyroid nodule. J Clin Endocrinol Metab. 1954 Jun;14(6):672–673. doi: 10.1210/jcem-14-6-672. [DOI] [PubMed] [Google Scholar]
- Rücker W., Schrör K. Evidence for high affinity prostacyclin binding sites in vascular tissue: radioligand studies with a chemically stable analogue. Biochem Pharmacol. 1983 Aug 15;32(16):2405–2410. doi: 10.1016/0006-2952(83)90683-4. [DOI] [PubMed] [Google Scholar]
- Sand G., Jortay A., Pochet R., Dumont J. E. Adenylate cyclase and protein phosphokinase activities in human thyroid. Comparison of normal glands, hyperfunctional nodules and carcinomas. Eur J Cancer. 1976 Jun;12(6):447–453. doi: 10.1016/0014-2964(76)90034-7. [DOI] [PubMed] [Google Scholar]
- Shenkman L., Imai Y., Kataoka K., Hollander C. S., Wan L., Tang S. C., AvRuskin T. Prostaglandins stimulate thyroid function in pregnant women. Science. 1974 Apr 5;184(4132):81–82. doi: 10.1126/science.184.4132.81. [DOI] [PubMed] [Google Scholar]
- Skuballa W., Vorbrüggen H. Synthesis of ciloprost (ZK 36 374): a chemically stable and biologically potent prostacyclin analog. Adv Prostaglandin Thromboxane Leukot Res. 1983;11:299–305. [PubMed] [Google Scholar]
- Takasu N., Kubota T., Ujiie A., Hamano S., Yamada T., Shimizu Y. Augmentation of prostacyclin and depression of PGE2, PGF2 alpha and thromboxane A2 by TSH in cultured porcine thyroid cells: an important role of prostacyclin in maintaining thyroid cell function. FEBS Lett. 1981 Apr 20;126(2):301–305. doi: 10.1016/0014-5793(81)80266-9. [DOI] [PubMed] [Google Scholar]
- Takasu N., Sato S., Yamada T., Makiuchi M., Furihata R. The different modes of action of thyrotropin and prostaglandin E1 on cyclic adenosine 3',5'-monophosphate synthesis in human thyroid, as studied by sequential stimulations. Horm Metab Res. 1976 May;8(3):206–211. doi: 10.1055/s-0028-1093661. [DOI] [PubMed] [Google Scholar]


