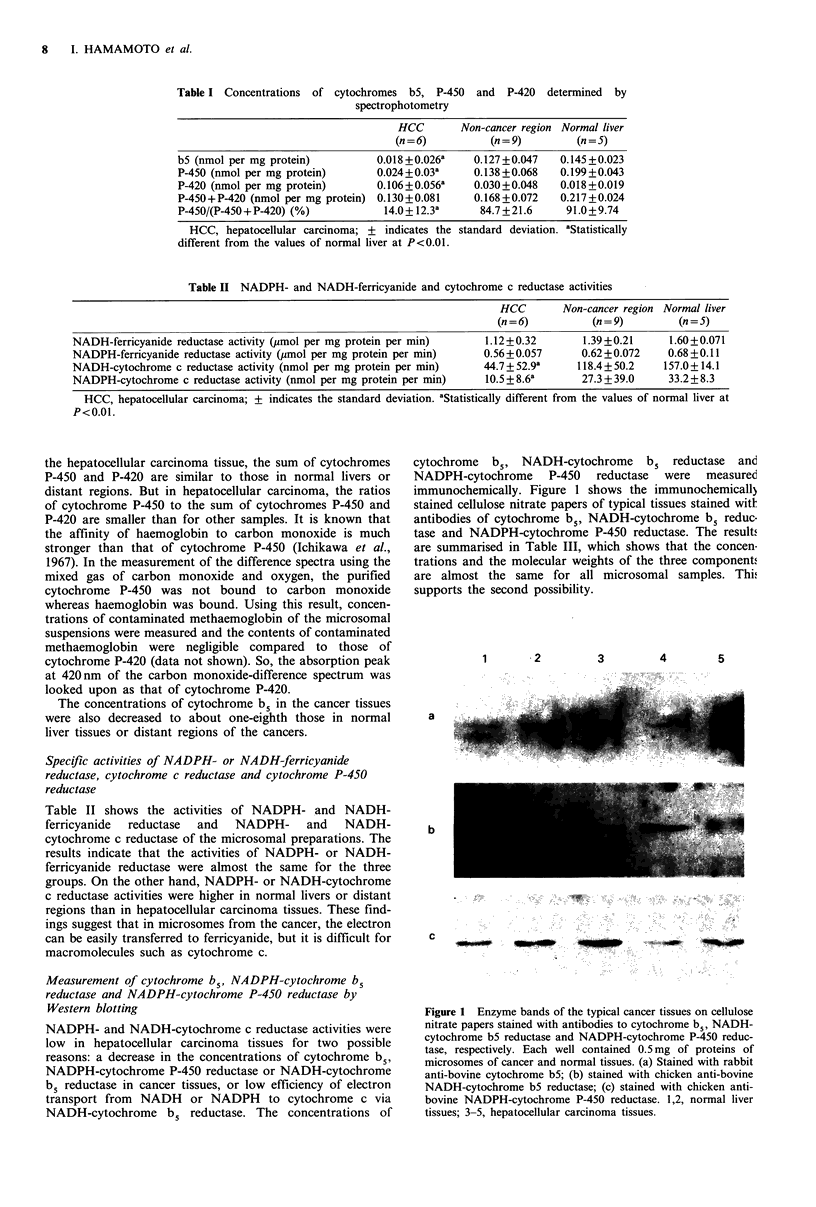
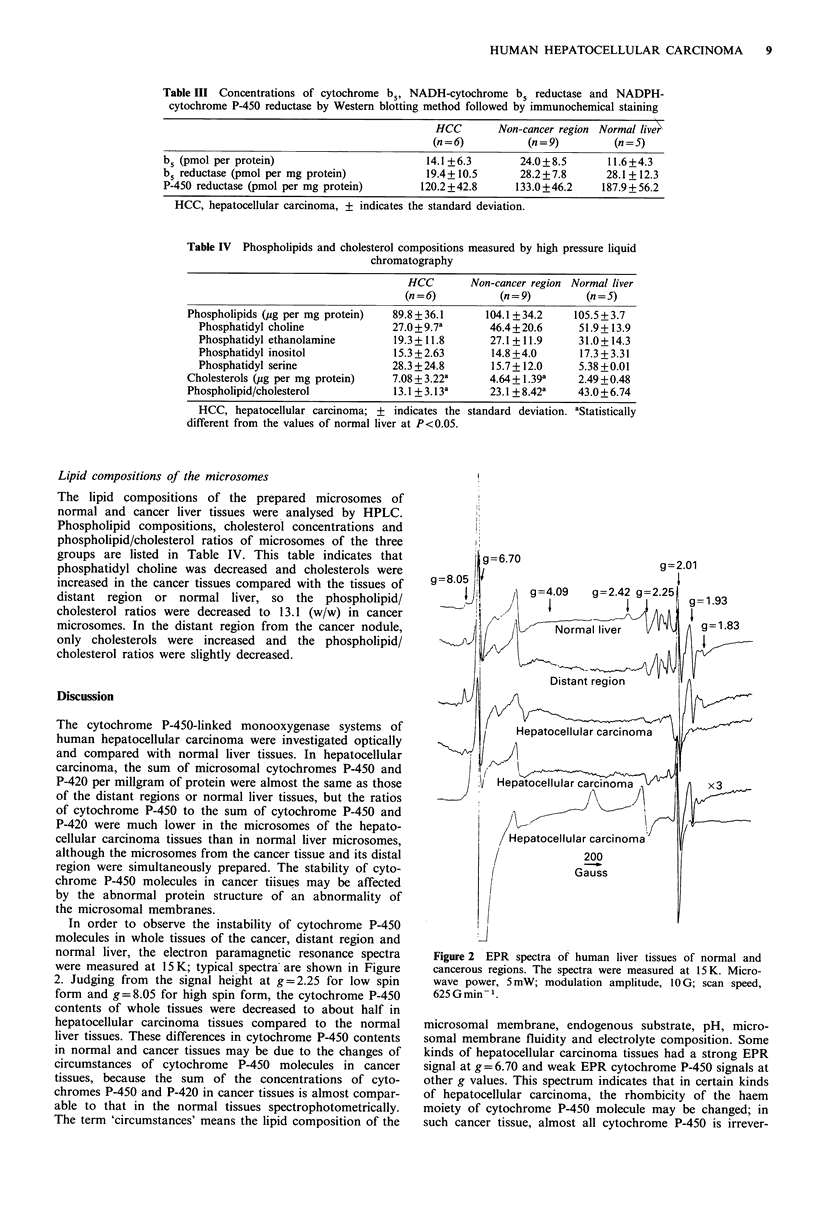
Abstract
The tissues of hepatocellular carcinoma were operatively resected from six patients. All four components of the systems of microsomal cytochrome P-450-linked monooxygenase of the tissues were investigated and compared to those of normal liver tissue. The concentrations of cytochromes P-450, P-420 and b5 were measured optically and the concentrations of all components except cytochrome P-450 were measured by the Western blotting method followed by immunochemical staining. In microsomes of hepatocellular carcinoma tissues, there was as much cytochrome P-450 and other redox components as in the normal liver tissues, but cytochrome P-450 in liver cancer tissues was unstable and easily converted to cytochrome P-420. The specific activities of NADPH- and NADH-ferricyanide and cytochrome c reductase of each sample were also measured. In the microsomes of the cancer tissues, the specific activities were remarkably reduced compared with those of normal liver tissues. The lipid compositions of the microsomes and the phospholipid/cholesterol ratios (w/w) were 13.1 +/- 3.13 in the cancer tissues and 43.0 +/- 6.74 in normal liver tissues. This difference of the lipid composition elucidates the instability of cytochrome P-450 molecules and the inefficiency of the electron transport of cytochrome P-450-linked monooxygenase systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aström A., DePierre J. W., Eriksson L. Characterization of drug-metabolizing systems in hyperplastic nodules from the livers of rats receiving 2-acetylaminofluorene in their diet. Carcinogenesis. 1983;4(5):577–581. doi: 10.1093/carcin/4.5.577. [DOI] [PubMed] [Google Scholar]
- Benga G., Pop V. I., Ionescu M., Hodârnău A., Tilinca R., Frangopol P. T. Comparison of human and rat liver microsomes by spin label and biochemical analyses. Biochim Biophys Acta. 1983 Jan 7;750(1):194–199. doi: 10.1016/0005-2760(83)90220-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- COOPER D. Y., LEVIN S., NARASIMHULU S., ROSENTHAL O. PHOTOCHEMICAL ACTION SPECTRUM OF THE TERMINAL OXIDASE OF MIXED FUNCTION OXIDASE SYSTEMS. Science. 1965 Jan 22;147(3656):400–402. doi: 10.1126/science.147.3656.400. [DOI] [PubMed] [Google Scholar]
- Eriksson L. C., Torndal U. B., Andersson G. N. Isolation and characterization of endoplasmic reticulum and Golgi apparatus from hepatocyte nodules in male wistar rats. Cancer Res. 1983 Jul;43(7):3335–3347. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Farber E. The biochemistry of preneoplastic liver: a common metabolic pattern in hepatocyte nodules. Can J Biochem Cell Biol. 1984 Jun;62(6):486–494. doi: 10.1139/o84-066. [DOI] [PubMed] [Google Scholar]
- Fiehn W., Hasselbach W. The effect of phospholipase A on the calcium transport and the role of unsaturated fatty acids in ATPase activity of sarcoplasmic vesicles. Eur J Biochem. 1970 Apr;13(3):510–518. doi: 10.1111/j.1432-1033.1970.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Hamamoto I., Hiwatashi A., Ichikawa Y. Zonal distribution of cytochromes P-450 and related enzymes of bovine adrenal cortex--quantitative assay of concentrations and total contents. J Biochem. 1986 Jun;99(6):1743–1748. doi: 10.1093/oxfordjournals.jbchem.a135651. [DOI] [PubMed] [Google Scholar]
- Hiwatashi A., Ichikawa Y., Maruya N., Yamano T., Aki K. Properties of crystalline reduced nicotinamide adenine dinucleotide phosphate-adrenodoxin reductase from bovine adrenocortical mitochonria. I. Physicochemical properties of holo- and apo-NADPH-adrenodoxin reductase and interaction between non-heme iron proteins and the reductase. Biochemistry. 1976 Jul 13;15(14):3082–3090. doi: 10.1021/bi00659a023. [DOI] [PubMed] [Google Scholar]
- Hrycay E. G., Prough R. A. Reduced nicotinamide adenine dinucleotide-cytochrome b5 reductase and cytochrome b5 as electron carriers in NADH-supported cytochrome P-450 -dependent enzyme activities in liver microsomes. Arch Biochem Biophys. 1974 Nov;165(1):331–339. doi: 10.1016/0003-9861(74)90171-4. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y., Hagihara B., Yamano T. Magnetic and spectrophotometric properties of the microsomal carbon monoxide binding pigment. Arch Biochem Biophys. 1967 Apr;120(1):204–213. doi: 10.1016/0003-9861(67)90615-7. [DOI] [PubMed] [Google Scholar]
- Ichikawa Y., Yamano T. Preparation and physicochemical properties of functional hemoprotein P450 from mammalian tissue microsomes. Biochim Biophys Acta. 1970 Feb 17;200(2):220–240. doi: 10.1016/0005-2795(70)90166-2. [DOI] [PubMed] [Google Scholar]
- Iyanagi T., Mason H. S. Some properties of hepatic reduced nicotinamide adenine dinucleotide phosphate-cytochrome c reductase. Biochemistry. 1973 Jun 5;12(12):2297–2308. doi: 10.1021/bi00736a018. [DOI] [PubMed] [Google Scholar]
- Kaduce T. L., Norton K. C., Spector A. A. A rapid, isocratic method for phospholipid separation by high-performance liquid chromatography. J Lipid Res. 1983 Oct;24(10):1398–1403. [PubMed] [Google Scholar]
- Kapitulnik J., Weil E., Rabinowitz R., Krausz M. M. Fetal and adult human liver differ markedly in the fluidity and lipid composition of their microsomal membranes. Hepatology. 1987 Jan-Feb;7(1):55–60. doi: 10.1002/hep.1840070113. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Norman R. L., Muller-Eberhard U., Johnson E. F. The role of cytochrome P-450 forms in 2-aminoanthracene and benz[alpha]pyrene mutagenesis. Biochem Biophys Res Commun. 1979 Jul 12;89(1):195–201. doi: 10.1016/0006-291x(79)90963-x. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Oprian D. D., Coon M. J. Oxidation-reduction states of FMN and FAD in NADPH-cytochrome P-450 reductase during reduction by NADPH. J Biol Chem. 1982 Aug 10;257(15):8935–8944. [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of reduced nicotinamide adenine dinucleotide-cytochrome b 5 reductase containing both the catalytic site and an additional hydrophobic membrane-binding segment. J Biol Chem. 1973 Feb 10;248(3):793–799. [PubMed] [Google Scholar]
- Strobel H. W., Lu A. Y., Heidema J., Coon M. J. Phosphatidylcholine requirement in the enzymatic reduction of hemoprotein P-450 and in fatty acid, hydrocarbon, and drug hydroxylation. J Biol Chem. 1970 Sep 25;245(18):4851–4854. [PubMed] [Google Scholar]
- Wang P. P., Beaune P., Kaminsky L. S., Dannan G. A., Kadlubar F. F., Larrey D., Guengerich F. P. Purification and characterization of six cytochrome P-450 isozymes from human liver microsomes. Biochemistry. 1983 Nov 8;22(23):5375–5383. doi: 10.1021/bi00292a019. [DOI] [PubMed] [Google Scholar]
- Waskell L., Koblin D., Canova-Davis E. The lipid composition of human liver microsomes. Lipids. 1982 Apr;17(4):317–320. doi: 10.1007/BF02534948. [DOI] [PubMed] [Google Scholar]
- Wrighton S. A., Campanile C., Thomas P. E., Maines S. L., Watkins P. B., Parker G., Mendez-Picon G., Haniu M., Shively J. E., Levin W. Identification of a human liver cytochrome P-450 homologous to the major isosafrole-inducible cytochrome P-450 in the rat. Mol Pharmacol. 1986 Apr;29(4):405–410. [PubMed] [Google Scholar]
- el Mouelhi M., Didolkar M. S., Elias E. G., Guengerich F. P., Kauffman F. C. Hepatic drug-metabolizing enzymes in primary and secondary tumors of human liver. Cancer Res. 1987 Jan 15;47(2):460–466. [PubMed] [Google Scholar]