Abstract
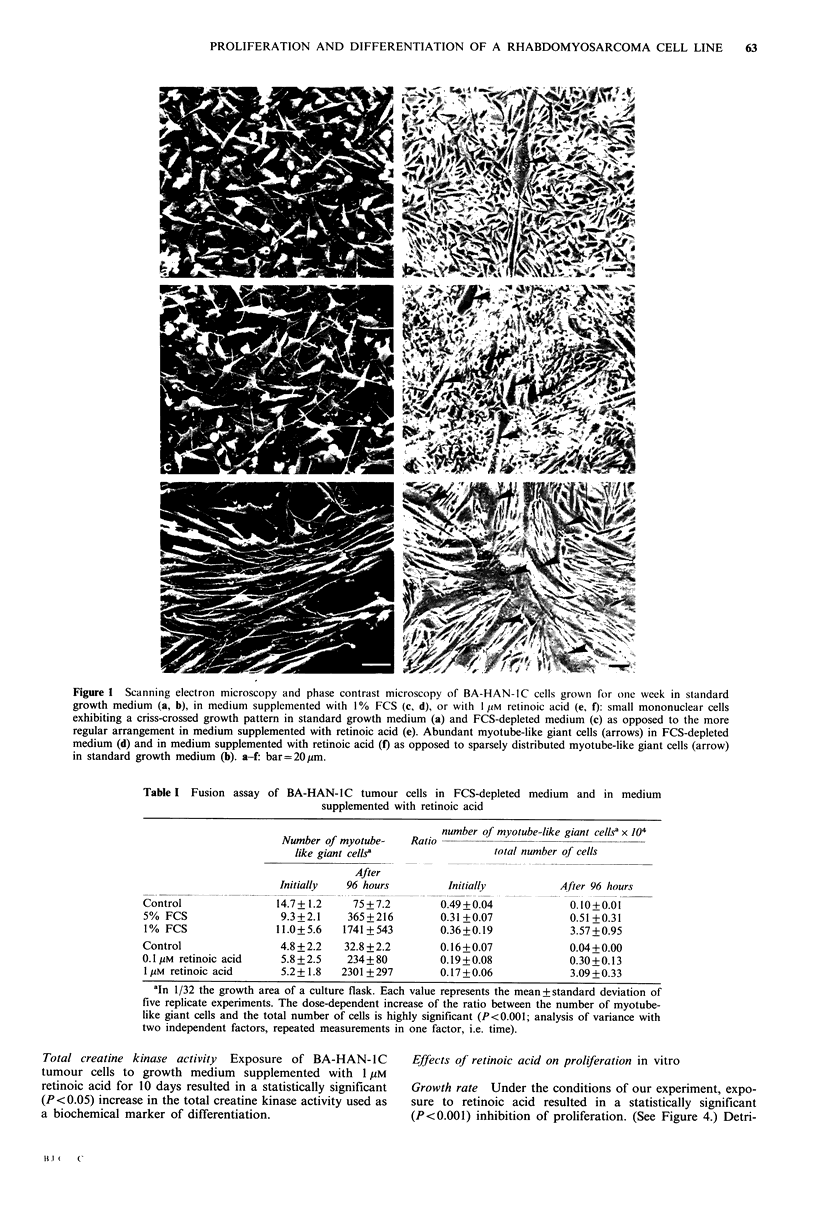
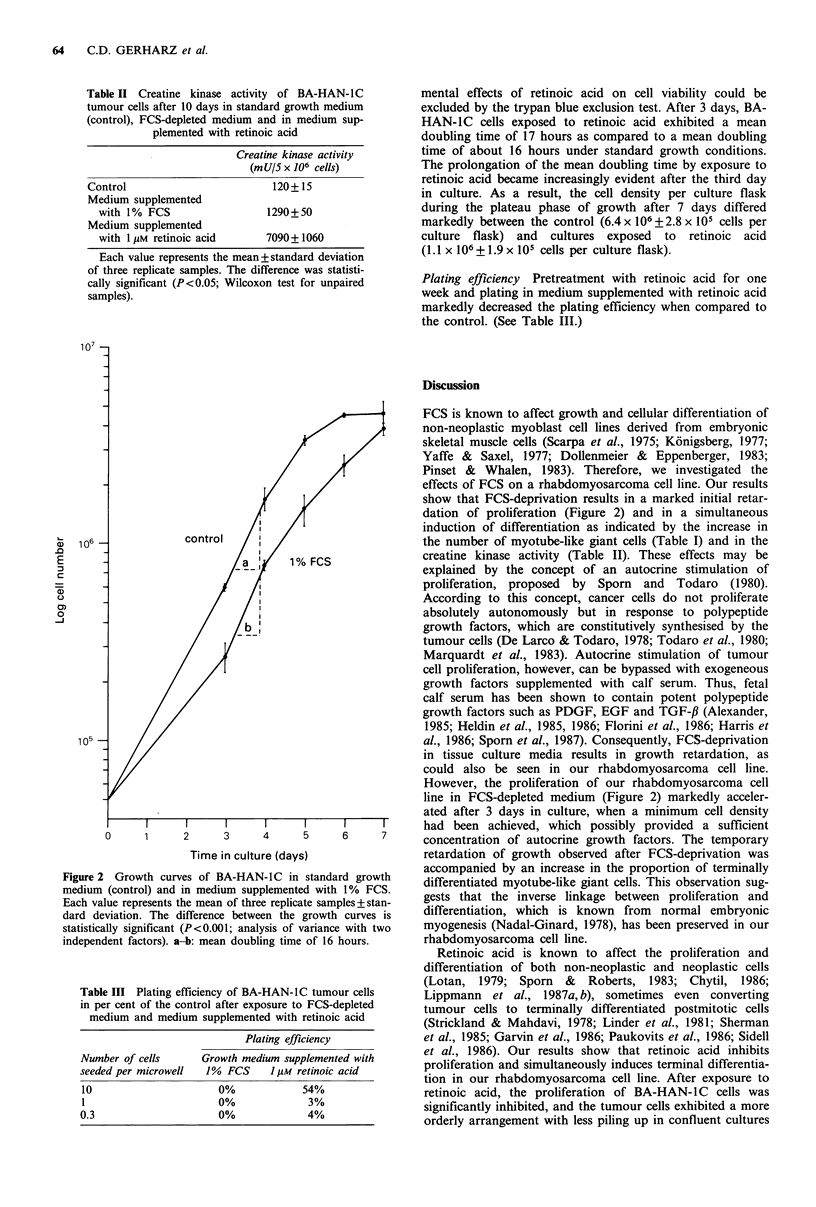
We report on the establishment of a model for differentiation induction in sarcomas, using the clonal rhabdomyosarcoma cell line BA-HAN-1C. This rhabdomyosarcoma cell line is composed of morphologically undifferentiated mononuclear stem cells, some of which spontaneously fuse to form terminally differentiated multinuclear myotube-like giant cells. The deprivation of fetal calf serum (FCS) or the exposure to retinoic acid, respectively, resulted in a significant inhibition of proliferation (P less than 0.001) and a marked increase in cellular differentiation as shown by a significant increase in the number of myotube-like giant cells (P less than 0.001) and in the creatine kinase activity (P less than 0.05) used as a biochemical marker of differentiation. Furthermore, after exposure to retinoic acid about 30% of the mononuclear tumour cells exhibited morphological features of rhabdomyogenic differentiation, such as bundles of thick and thin myofilaments, which had never been observed in the mononuclear cells of untreated cultures. These results confirm that the inverse linkage between proliferation and differentiation known from embryonic myogenesis is preserved in our rhabdomyosarcoma cell line. The failure to induce terminal differentiation by exposure to retinoic acid in all the cells of our clonal cell line indicates that some tumour cells might epigenetically be blocked from responding to retinoic acid. The temporary growth retardation observed after FCS-deprivation suggests that autocrine stimulation of proliferation may be operating in our cell line, too.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P. Do cancers arise from a single transformed cell or is monoclonality of tumours a late event in carcinogenesis? Br J Cancer. 1985 Apr;51(4):453–457. doi: 10.1038/bjc.1985.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatruda T. T., 3rd, Sidell N., Ranyard J., Koeffler H. P. Retinoic acid treatment of human neuroblastoma cells is associated with decreased N-myc expression. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1189–1195. doi: 10.1016/0006-291x(85)90311-0. [DOI] [PubMed] [Google Scholar]
- Annesley T., Giacherio D., Wilkerson K., Grekin R., Ellis C. Analysis of retinoids by high-performance liquid chromatography using programmed gradient separation. J Chromatogr. 1984 Jan 13;305(1):199–203. doi: 10.1016/s0378-4347(00)83330-7. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12;321(6071):702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Delaporte C., Dautreaux B., Fardeau M. Human myotube differentiation in vitro in different culture conditions. Biol Cell. 1986;57(1):17–22. doi: 10.1111/j.1768-322x.1986.tb00460.x. [DOI] [PubMed] [Google Scholar]
- Dexter D. L. N,N-Dimethylformamide-induced morphological differentiation and reduction of tumorigenicity in cultured mouse rhabdomyosarcoma cells. Cancer Res. 1977 Sep;37(9):3136–3140. [PubMed] [Google Scholar]
- Florini J. R., Roberts A. B., Ewton D. Z., Falen S. L., Flanders K. C., Sporn M. B. Transforming growth factor-beta. A very potent inhibitor of myoblast differentiation, identical to the differentiation inhibitor secreted by Buffalo rat liver cells. J Biol Chem. 1986 Dec 15;261(35):16509–16513. [PubMed] [Google Scholar]
- Freshney R. I. Induction of differentiation in neoplastic cells. Anticancer Res. 1985 Jan-Feb;5(1):111–130. [PubMed] [Google Scholar]
- Gabbert H. E., Gerharz C. D., Engers R., Müller-Klieser W., Moll R. Terminally differentiated postmitotic tumor cells in a rat rhabdomyosarcoma cell line. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;55(5):255–261. doi: 10.1007/BF02896584. [DOI] [PubMed] [Google Scholar]
- Garvin A. J., Stanley W. S., Bennett D. D., Sullivan J. L., Sens D. A. The in vitro growth, heterotransplantation, and differentiation of a human rhabdomyosarcoma cell line. Am J Pathol. 1986 Oct;125(1):208–217. [PMC free article] [PubMed] [Google Scholar]
- Gerharz C. D., Gabbert H., Moll R., Mellin W., Engers R., Gabbiani G. The intraclonal and interclonal phenotypic heterogeneity in a rhabdomyosarcoma cell line with abortive imitation of embryonic myogenesis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;55(4):193–206. doi: 10.1007/BF02896576. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Platelet-derived growth factor. Mol Cell Endocrinol. 1985 Mar;39(3):169–187. doi: 10.1016/0303-7207(85)90061-9. [DOI] [PubMed] [Google Scholar]
- Jetten A. M. Retinoids specifically enhance the number of epidermal growth factor receptors. Nature. 1980 Apr 17;284(5757):626–629. doi: 10.1038/284626a0. [DOI] [PubMed] [Google Scholar]
- Linder S., Krondahl U., Sennerstam R., Ringertz N. R. Retinoic acid-induced differentiation of F9 embryonal carcinoma cells. Exp Cell Res. 1981 Apr;132(2):453–460. doi: 10.1016/0014-4827(81)90120-8. [DOI] [PubMed] [Google Scholar]
- Lippman S. M., Kessler J. F., Meyskens F. L., Jr Retinoids as preventive and therapeutic anticancer agents (Part I). Cancer Treat Rep. 1987 Apr;71(4):391–405. [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Twardzik D. R., De Larco J. E., Stephenson J. R., Todaro G. J. Transforming growth factors produced by retrovirus-transformed rodent fibroblasts and human melanoma cells: amino acid sequence homology with epidermal growth factor. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4684–4688. doi: 10.1073/pnas.80.15.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. E., Mitra N. S., Moritz E. A. Differentiation of F9 embryonal carcinoma cells. Differences in the effects of retinoic acid, 5-bromodeoxyuridine, and N'-N'-dimethylacetamide. Differentiation. 1986;31(3):183–190. doi: 10.1111/j.1432-0436.1986.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Paukovits J. B., Paukovits W. R., Laerum O. D. Identification of a regulatory peptide distinct from normal granulocyte-derived hemoregulatory peptide produced by human promyelocytic HL-60 leukemia cells after differentiation induction with retinoic acid. Cancer Res. 1986 Sep;46(9):4444–4448. [PubMed] [Google Scholar]
- Pierce G. B. Neoplasms, differentiations and mutations. Am J Pathol. 1974 Oct;77(1):103–118. [PMC free article] [PubMed] [Google Scholar]
- Sachs L. Cell differentiation and bypassing of genetic defects in the suppression of malignancy. Cancer Res. 1987 Apr 15;47(8):1981–1986. [PubMed] [Google Scholar]
- Sachs L. Constitutive uncoupling of pathways of gene expression that control growth and differentiation in myeloid leukemia: a model for the origin and progression of malignancy. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6152–6156. doi: 10.1073/pnas.77.10.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa S., Uhlendorf B. W., Cantoni G. L. The differentiation of L5/A10 myoblast cell line (a subclone of L5 line) is controlled by changes of culture conditions. Cell Differ. 1985 Aug;17(2):105–114. doi: 10.1016/0045-6039(85)90476-2. [DOI] [PubMed] [Google Scholar]
- Sherman M. I., Eglitis M. A., Thomas R. Reversible and irreversible effects of retinol upon the phenotypic properties of embryonal carcinoma cells. J Embryol Exp Morphol. 1986 Apr;93:179–196. [PubMed] [Google Scholar]
- Sidell N., Sarafian T., Kelly M., Tsuchida T., Haussler M. Retinoic acid-induced differentiation of human neuroblastoma: a cell variant system showing two distinct responses. Exp Cell Biol. 1986;54(5-6):287–300. doi: 10.1159/000163368. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983 Jul;43(7):3034–3040. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Todaro G. J. Autocrine secretion and malignant transformation of cells. N Engl J Med. 1980 Oct 9;303(15):878–880. doi: 10.1056/NEJM198010093031511. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Thiele C. J., Reynolds C. P., Israel M. A. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. 1985 Jan 31-Feb 6Nature. 313(6001):404–406. doi: 10.1038/313404a0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Fryling C., De Larco J. E. Transforming growth factors produced by certain human tumor cells: polypeptides that interact with epidermal growth factor receptors. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5258–5262. doi: 10.1073/pnas.77.9.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7(3):159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]
- Zile M. H., Cullum M. E., Simpson R. U., Barua A. B., Swartz D. A. Induction of differentiation of human promyelocytic leukemia cell line HL-60 by retinoyl glucuronide, a biologically active metabolite of vitamin A. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2208–2212. doi: 10.1073/pnas.84.8.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]