Abstract
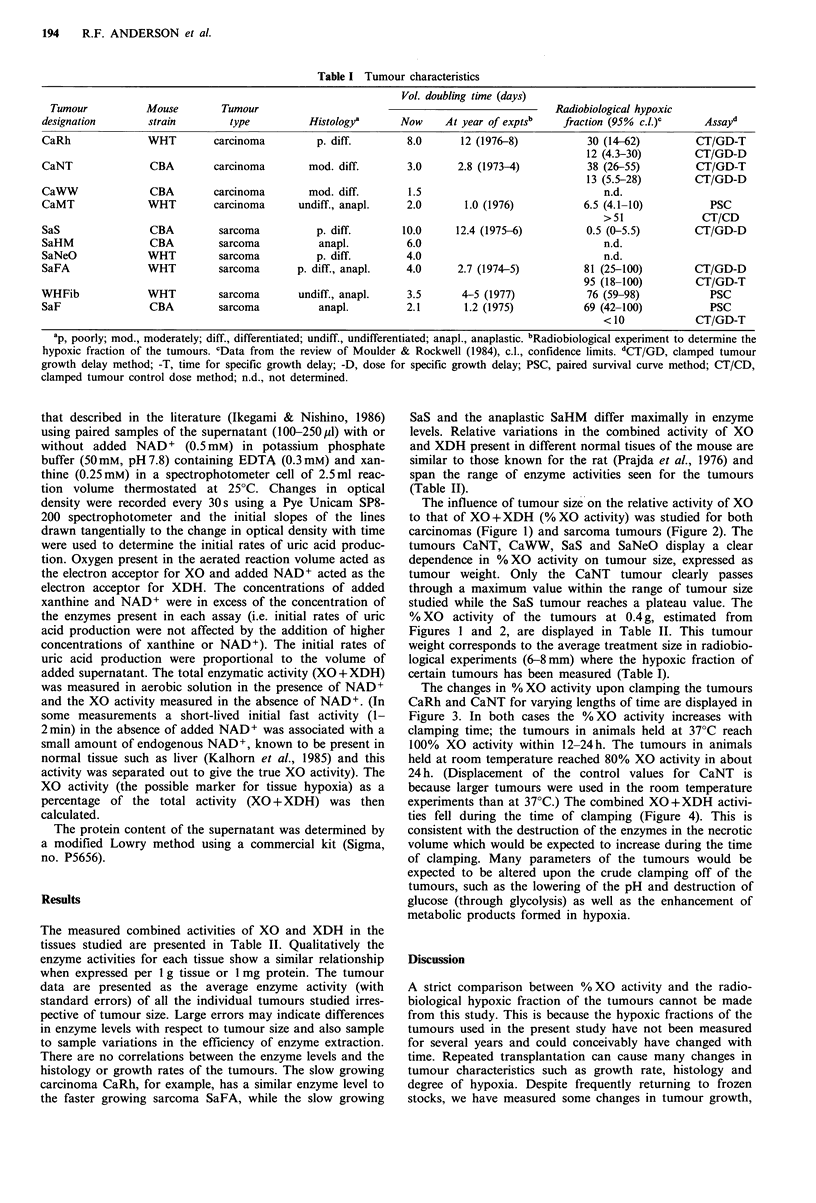
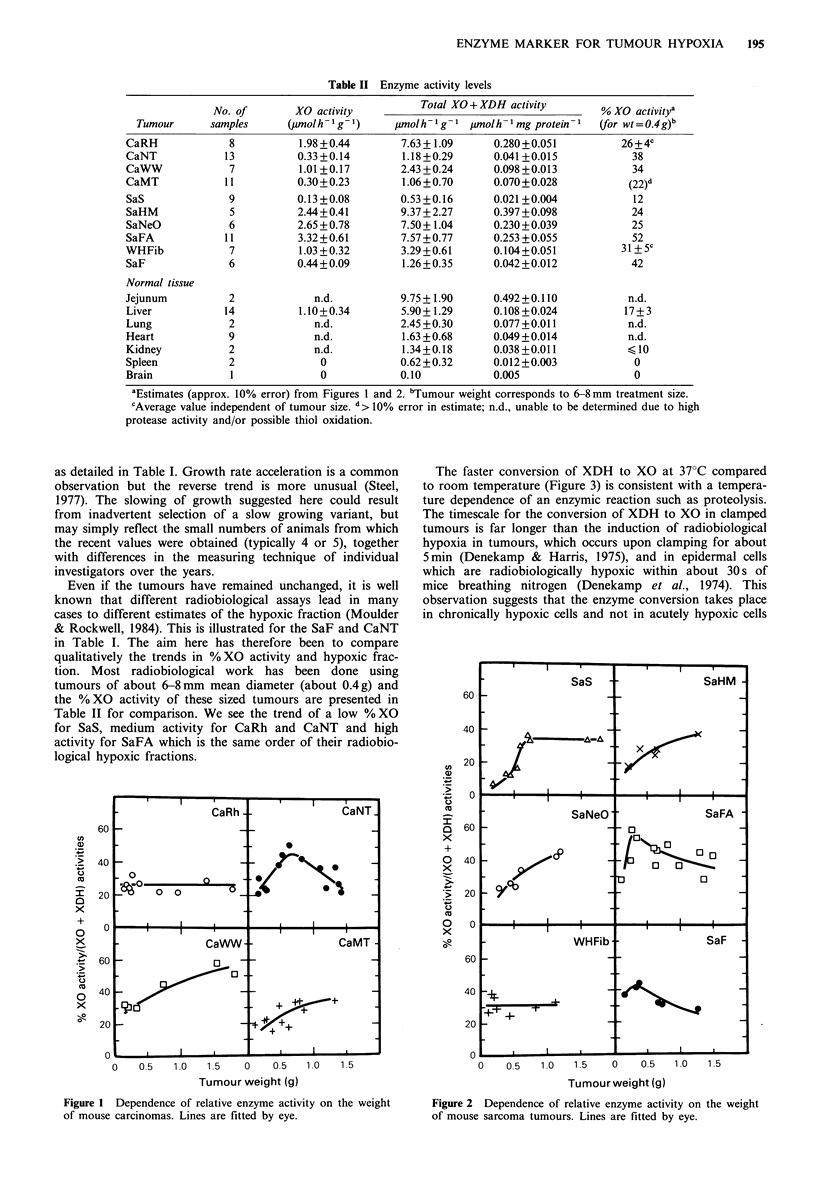
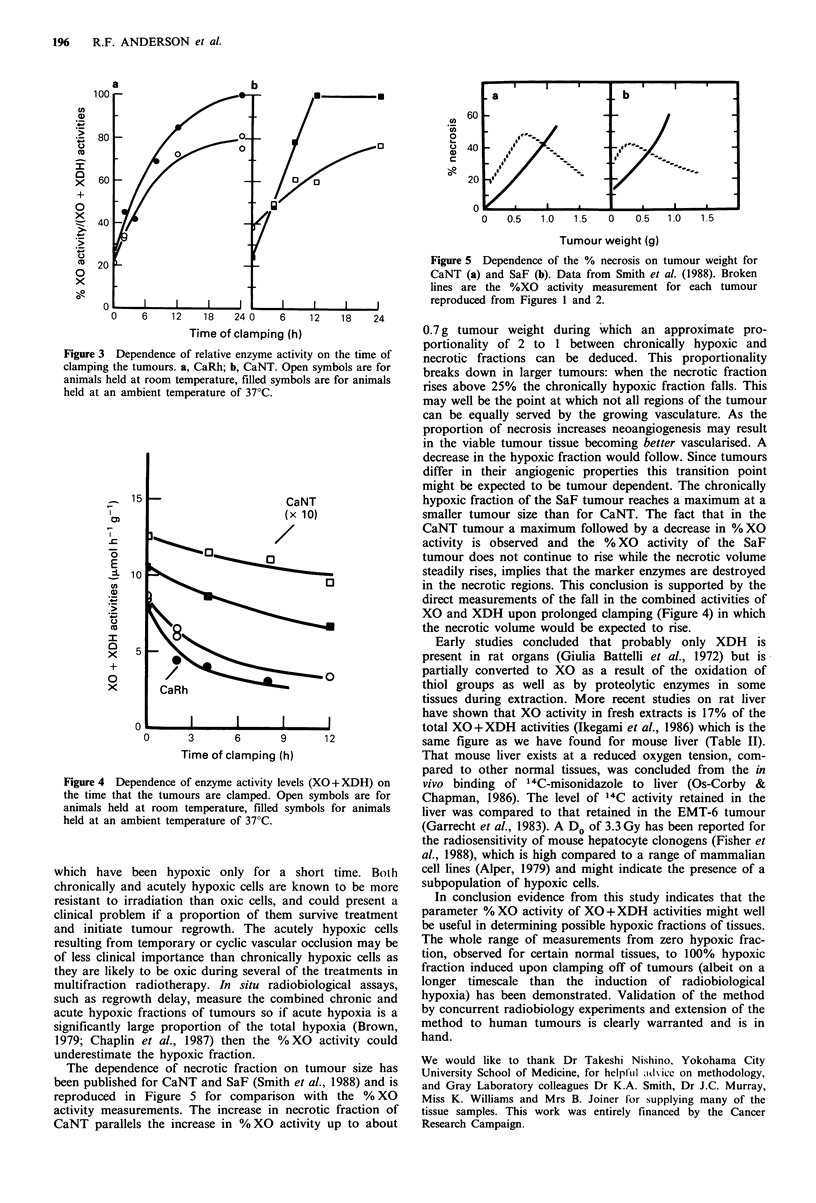
The enzyme activities of endogenous xanthine dehydrogenase (XDH) and xanthine oxidase (XO) have been measured in 10 different types of mouse tumour and seven normal tissues. The conversion of XDH to XO has been observed in two tumour types upon the prolonged clamping off of the blood supply to the tumours. It is proposed that a similar conversion might also occur naturally in chronically hypoxic cells and that the ratio of the XO activity to the combined XO + XDH activities (%XO activity) could well serve as a marker for tissue hypoxia. A qualitative relationship exists between the %XO activity and literature values of the hypoxic fraction for some tumours measured by radiobiological assays. The influence of tumour size (about 0.2-1.8 g) on %XO activity is presented for all 10 tumours as well as %XO activity determinations for four of the normal tissues.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battelli M. G., Corte E. D., Stirpe F. Xanthine oxidase type D (dehydrogenase) in the intestine and other organs of the rat. Biochem J. 1972 Feb;126(3):747–749. doi: 10.1042/bj1260747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M. Evidence for acutely hypoxic cells in mouse tumours, and a possible mechanism of reoxygenation. Br J Radiol. 1979 Aug;52(620):650–656. doi: 10.1259/0007-1285-52-620-650. [DOI] [PubMed] [Google Scholar]
- Chaplin D. J., Olive P. L., Durand R. E. Intermittent blood flow in a murine tumor: radiobiological effects. Cancer Res. 1987 Jan 15;47(2):597–601. [PubMed] [Google Scholar]
- Corte E. D., Stirpe F. The regulation of rat liver xanthine oxidase. Involvement of thiol groups in the conversion of the enzyme activity from dehydrogenase (type D) into oxidase (type O) and purification of the enzyme. Biochem J. 1972 Feb;126(3):739–745. doi: 10.1042/bj1260739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denekamp J., Harris S. R. Tests of two electron-affinic radiosensitizers in vivo using regrowth of an experimental carcinoma. Radiat Res. 1975 Feb;61(2):191–203. [PubMed] [Google Scholar]
- Denekamp J., Hill S. A., Hobson B. Vascular occlusion and tumour cell death. Eur J Cancer Clin Oncol. 1983 Feb;19(2):271–275. doi: 10.1016/0277-5379(83)90426-1. [DOI] [PubMed] [Google Scholar]
- Denekamp J., Michael B. D., Harris S. R. Hypoxic cell radiosensitizers: comparative tests of some electron affinic compounds using epidermal cell survival in vivo. Radiat Res. 1974 Oct;60(1):119–132. [PubMed] [Google Scholar]
- Fisher D. R., Hendry J. H., Scott D. Long-term repair in vivo of colony-forming ability and chromosomal injury in X-irradiated mouse hepatocytes. Radiat Res. 1988 Jan;113(1):40–50. [PubMed] [Google Scholar]
- Garrecht B. M., Chapman J. D. The labelling of EMT-6 tumours in BALB/C mice with 14C-misonidazole. Br J Radiol. 1983 Oct;56(670):745–753. doi: 10.1259/0007-1285-56-670-745. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Rutili G., McCord J. M. Superoxide radicals in feline intestinal ischemia. Gastroenterology. 1981 Jul;81(1):22–29. [PubMed] [Google Scholar]
- Horowitz M., Blasberg R., Molnar P., Strong J., Kornblith P., Pleasants R., Fenstermacher J. Regional [14C]misonidazole distribution in experimental RT-9 brain tumors. Cancer Res. 1983 Aug;43(8):3800–3807. [PubMed] [Google Scholar]
- Ikegami T., Natsumeda Y., Weber G. Decreased concentration of xanthine dehydrogenase (EC 1.1.1.204) in rat hepatomas. Cancer Res. 1986 Aug;46(8):3838–3841. [PubMed] [Google Scholar]
- Ikegami T., Nishino T. The presence of desulfo xanthine dehydrogenase in purified and crude enzyme preparations from rat liver. Arch Biochem Biophys. 1986 Jun;247(2):254–260. doi: 10.1016/0003-9861(86)90582-5. [DOI] [PubMed] [Google Scholar]
- Kalhorn T. F., Thummel K. E., Nelson S. D., Slattery J. T. Analysis of oxidized and reduced pyridine dinucleotides in rat liver by high-performance liquid chromatography. Anal Biochem. 1985 Dec;151(2):343–347. doi: 10.1016/0003-2697(85)90185-x. [DOI] [PubMed] [Google Scholar]
- Moulder J. E., Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984 May;10(5):695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- Prajda N., Morris H. P., Weber G. Imbalance of purine metabolism in hepatomas of different growth rates as expressed in behavior of xanthine oxidase (EC 1.2.3.2). Cancer Res. 1976 Dec;36(12):4639–4646. [PubMed] [Google Scholar]
- Smith K. A., Hill S. A., Begg A. C., Denekamp J. Validation of the fluorescent dye Hoechst 33342 as a vascular space marker in tumours. Br J Cancer. 1988 Mar;57(3):247–253. doi: 10.1038/bjc.1988.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urtasun R. C., Chapman J. D., Raleigh J. A., Franko A. J., Koch C. J. Binding of 3H-misonidazole to solid human tumors as a measure of tumor hypoxia. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1263–1267. doi: 10.1016/0360-3016(86)90273-7. [DOI] [PubMed] [Google Scholar]
- Van Os-Corby D. J., Chapman J. D. In vitro binding of 14C-misonidazole to hepatocytes and hepatoma cells. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1251–1254. doi: 10.1016/0360-3016(86)90270-1. [DOI] [PubMed] [Google Scholar]


