Abstract
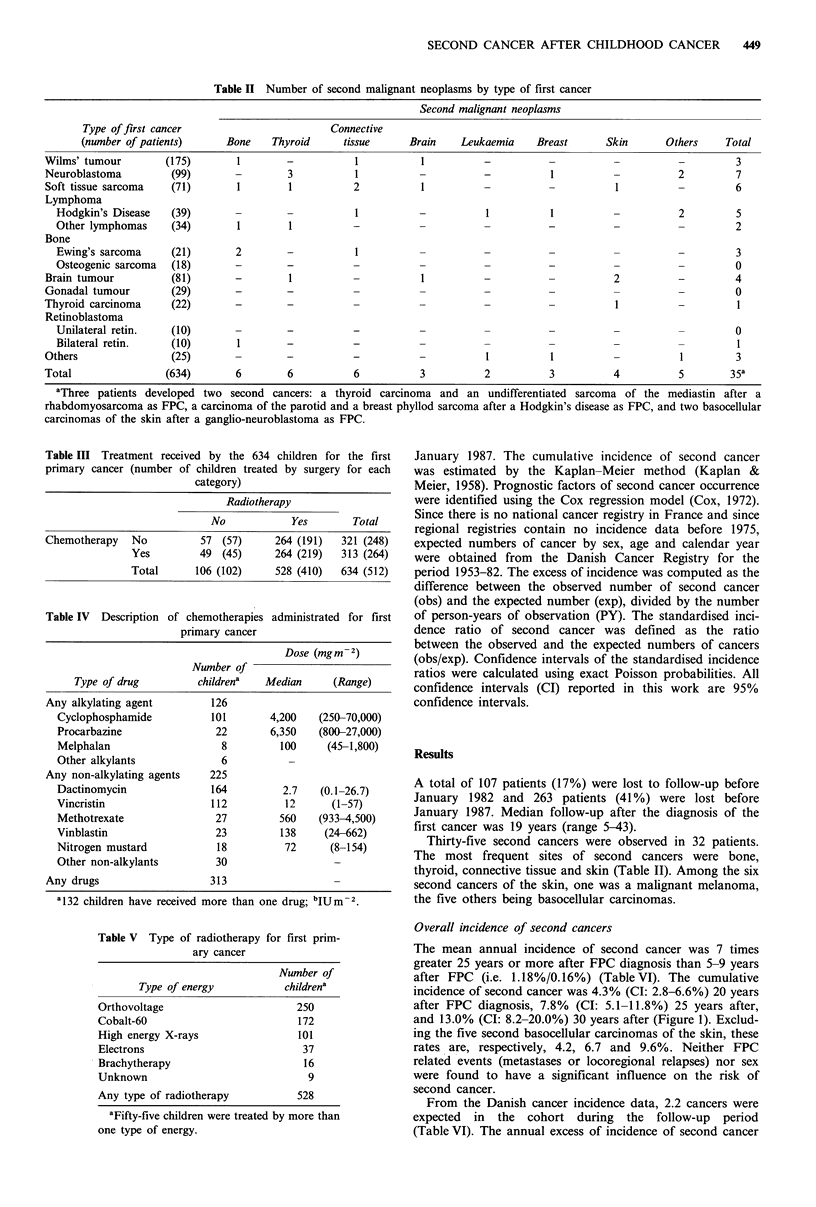
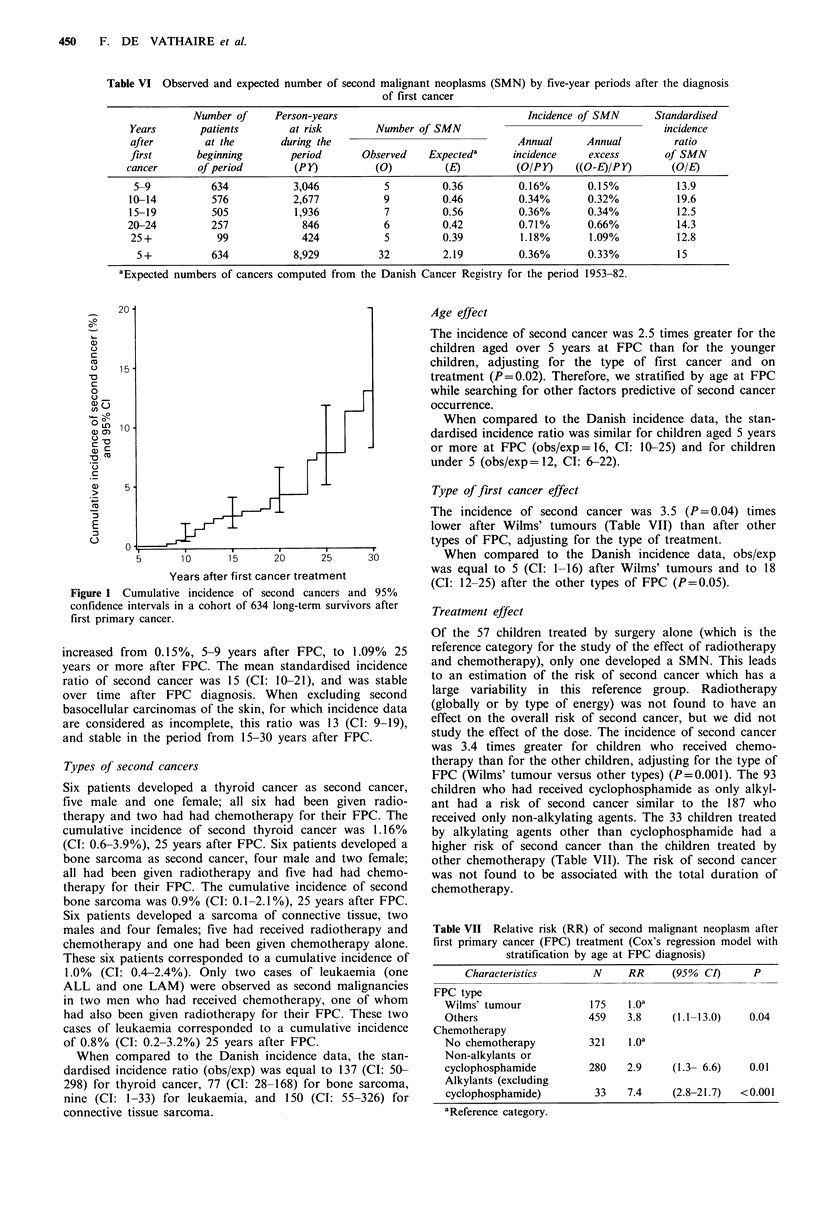
The risk of subsequent second malignant neoplasm was studied in a cohort of 634 patients, treated for a childhood cancer at the Gustave Roussy Institute between 1942 and 1969, and in complete remission five years after diagnosis. The most frequent types of first primary cancers (FPC) were Wilms' tumours (28% of the children), neuroblastomas (16%), lymphomas (12%) and soft tissue sarcomas (11%). Median follow-up duration after FPC was 19 years. Thirty-two patients (obs = 32) developed a total of 35 second cancers. Bone, thyroid, connective tissues and skin were the most frequent types of second cancer, with six patients for each type. The average annual incidence of second cancer was 0.36%. The average annual incidence for the periods 5-9, 10-14, 15-19, 20-24 and 25+ years after FPC was respectively 0.16%, 0.34%, 0.36%, 0.71% and 1.18%. The cumulative incidence of second cancer for the periods 5-20, 5-25 and 5-30 years after FPC was, respectively, 4.3% (95% CI: 2.8-6.6%), 7.8% (95% CI: 5.1-11.8%) and 13.0% (95% CI: 8.2-20.0%). The expected number of cancers in the cohort, computed from Danish cancer incidence data, was exp = 2.2. When compared to this expected number, the average annual excess incidence of second cancer, defined as obs-exp divided by the number of person years of observation, was 0.33%. This rose from 0.15% for the period 5-9 years after FPC to 1.09% for the period beginning 25 years after FPC. The standardised incidence ratio of second cancer (i.e. obs/exp) was 15 (95% CI: 10-21), and was fairly constant in the period extending from 15 to 20 years after FPC diagnosis. Obs/exp was equal to 25 for the patients who had had chemotherapy and equal to 9 for those who had not. Cyclophosphamide seemed less carcinogenic than the other alkylating agents. Obs/exp was similar for the patients who had received radiotherapy and for those who had not. The risk of cancer increased with age in the reference population and increased faster in the cohort, because the standardised incidence ratio is constant over a long period.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuzick J., Erskine S., Edelman D., Galton D. A. A comparison of the incidence of the myelodysplastic syndrome and acute myeloid leukaemia following melphalan and cyclophosphamide treatment for myelomatosis. A report to the Medical Research Council's working party on leukaemia in adults. Br J Cancer. 1987 May;55(5):523–529. doi: 10.1038/bjc.1987.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. H., Harris E. L., Gershenson D. M., Malkasian G. D., Jr, Melton L. J., 3rd, Dembo A. J., Bennett J. M., Moloney W. C., Boice J. D., Jr Melphalan may be a more potent leukemogen than cyclophosphamide. Ann Intern Med. 1986 Sep;105(3):360–367. doi: 10.7326/0003-4819-105-3-360. [DOI] [PubMed] [Google Scholar]
- Hawkins M. M., Draper G. J., Kingston J. E. Incidence of second primary tumours among childhood cancer survivors. Br J Cancer. 1987 Sep;56(3):339–347. doi: 10.1038/bjc.1987.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M. M. Second primary tumours among survivors of childhood cancer treated with anticancer drugs. IARC Sci Publ. 1986;(78):231–252. [PubMed] [Google Scholar]
- Li F. P., Cassady J. R., Jaffe N. Risk of second tumors in survivors of childhood cancer. Cancer. 1975 Apr;35(4):1230–1235. doi: 10.1002/1097-0142(197504)35:4<1230::aid-cncr2820350430>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Li F. P. Second malignant tumors after cancer in childhood. Cancer. 1977 Oct;40(4 Suppl):1899–1902. doi: 10.1002/1097-0142(197710)40:4+<1899::aid-cncr2820400821>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Li F. P., Yan J. C., Sallan S., Cassady J. R., Jr, Danahy J., Fine W., Gelber R. D., Green D. M. Second neoplasms after Wilms' tumor in childhood. J Natl Cancer Inst. 1983 Dec;71(6):1205–1209. [PubMed] [Google Scholar]
- Miké V., Meadows A. T., D'Angio G. J. Incidence of second malignant neoplasms in children: results of an international study. Lancet. 1982 Dec 11;2(8311):1326–1331. doi: 10.1016/s0140-6736(82)91524-0. [DOI] [PubMed] [Google Scholar]
- Olsen J. H. Risk of second cancer after cancer in childhood. Cancer. 1986 Jun 1;57(11):2250–2254. doi: 10.1002/1097-0142(19860601)57:11<2250::aid-cncr2820571131>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Potish R. A., Dehner L. P., Haselow R. E., Kim T. H., Levitt S. H., Nesbit M. The incidence of second neoplasms following megavoltage radiation for pediatric tumors. Cancer. 1985 Oct 1;56(7):1534–1537. doi: 10.1002/1097-0142(19851001)56:7<1534::aid-cncr2820560711>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Tucker M. A., D'Angio G. J., Boice J. D., Jr, Strong L. C., Li F. P., Stovall M., Stone B. J., Green D. M., Lombardi F., Newton W. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987 Sep 3;317(10):588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- Tucker M. A., Meadows A. T., Boice J. D., Jr, Stovall M., Oberlin O., Stone B. J., Birch J., Voûte P. A., Hoover R. N., Fraumeni J. F., Jr Leukemia after therapy with alkylating agents for childhood cancer. J Natl Cancer Inst. 1987 Mar;78(3):459–464. [PubMed] [Google Scholar]


