Abstract
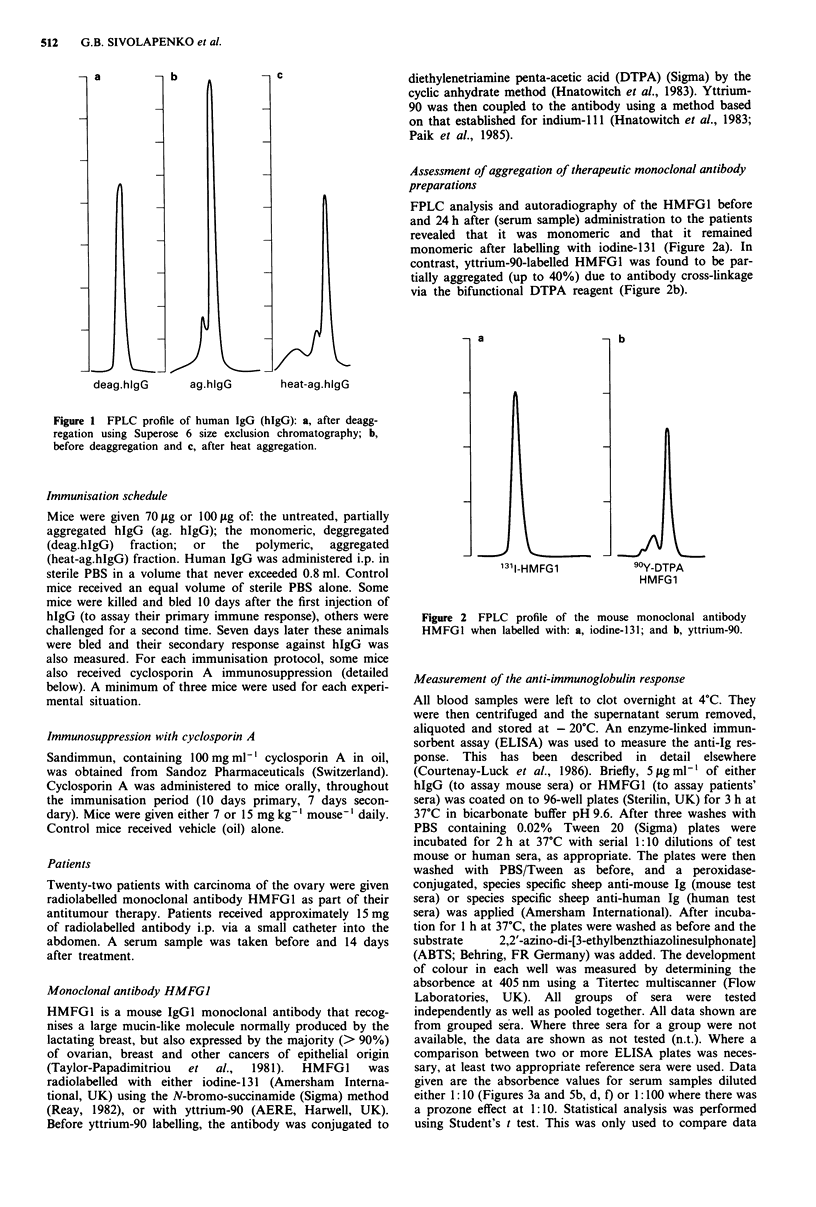
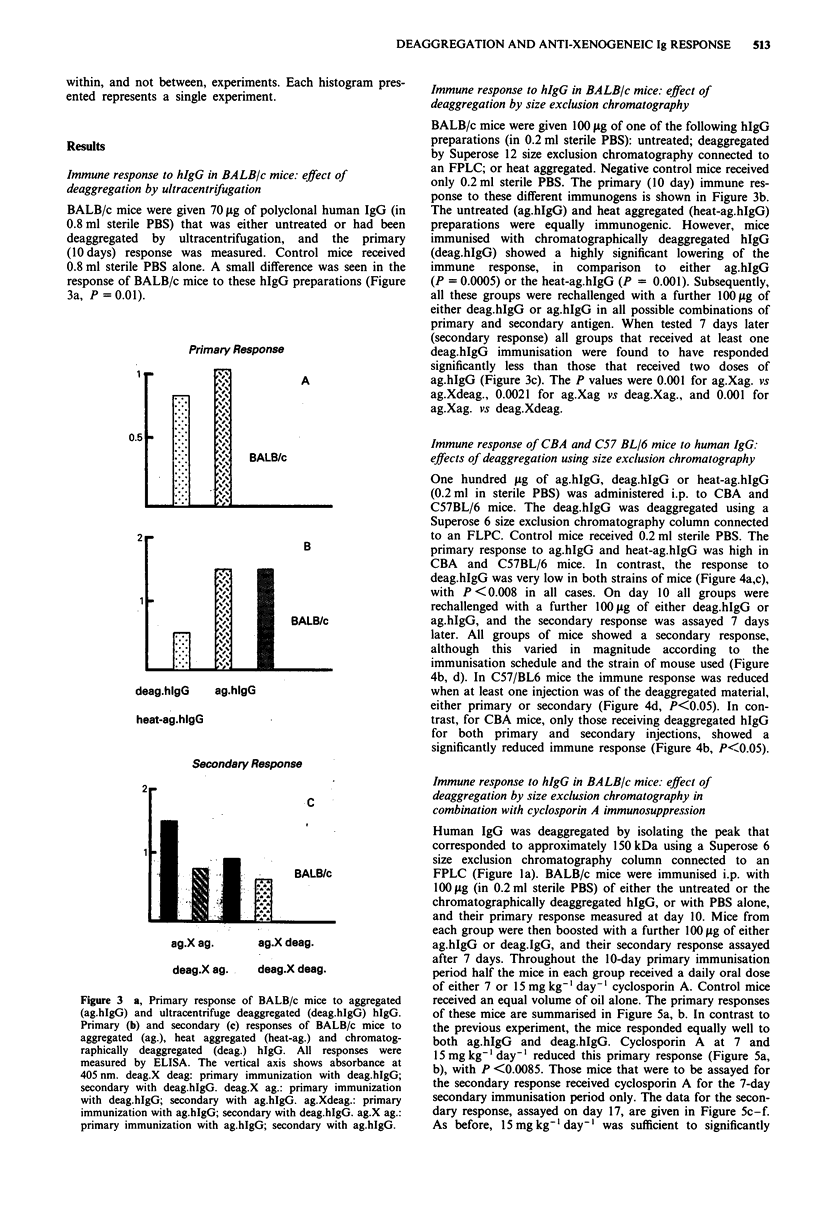
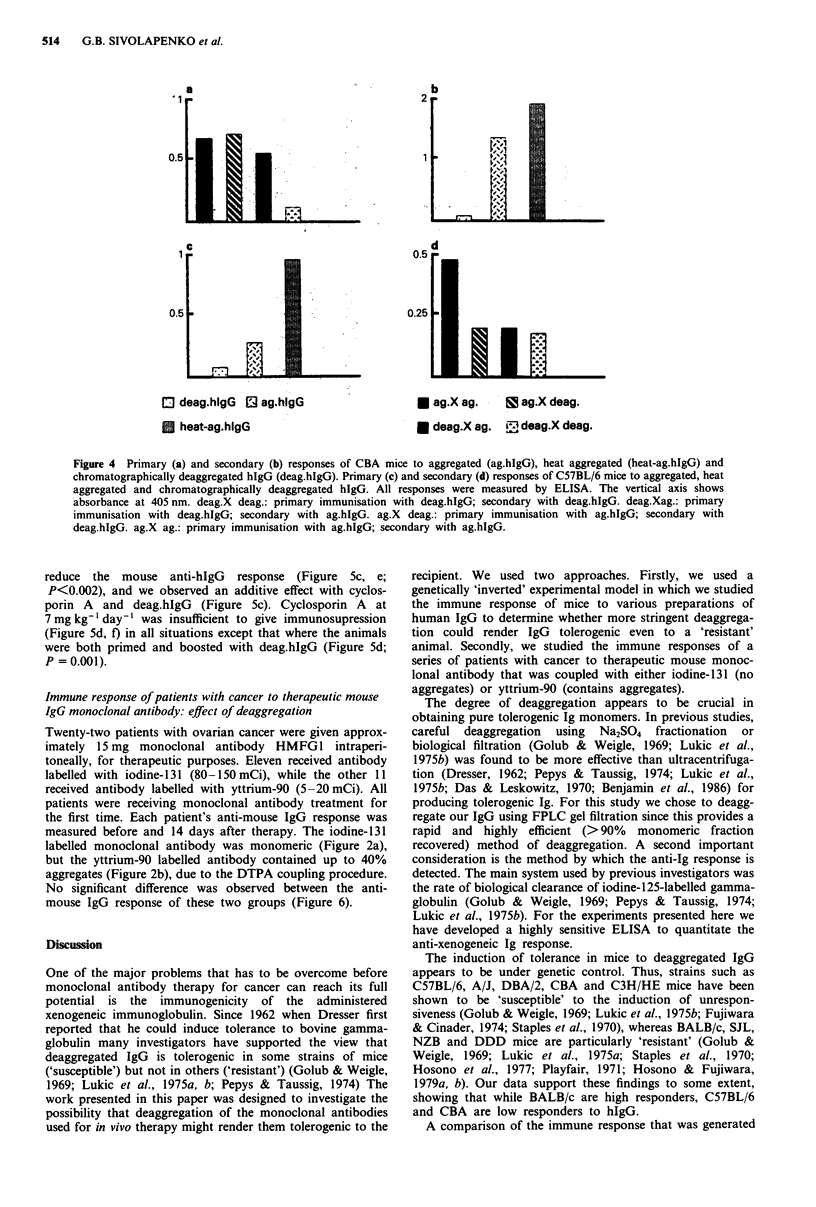
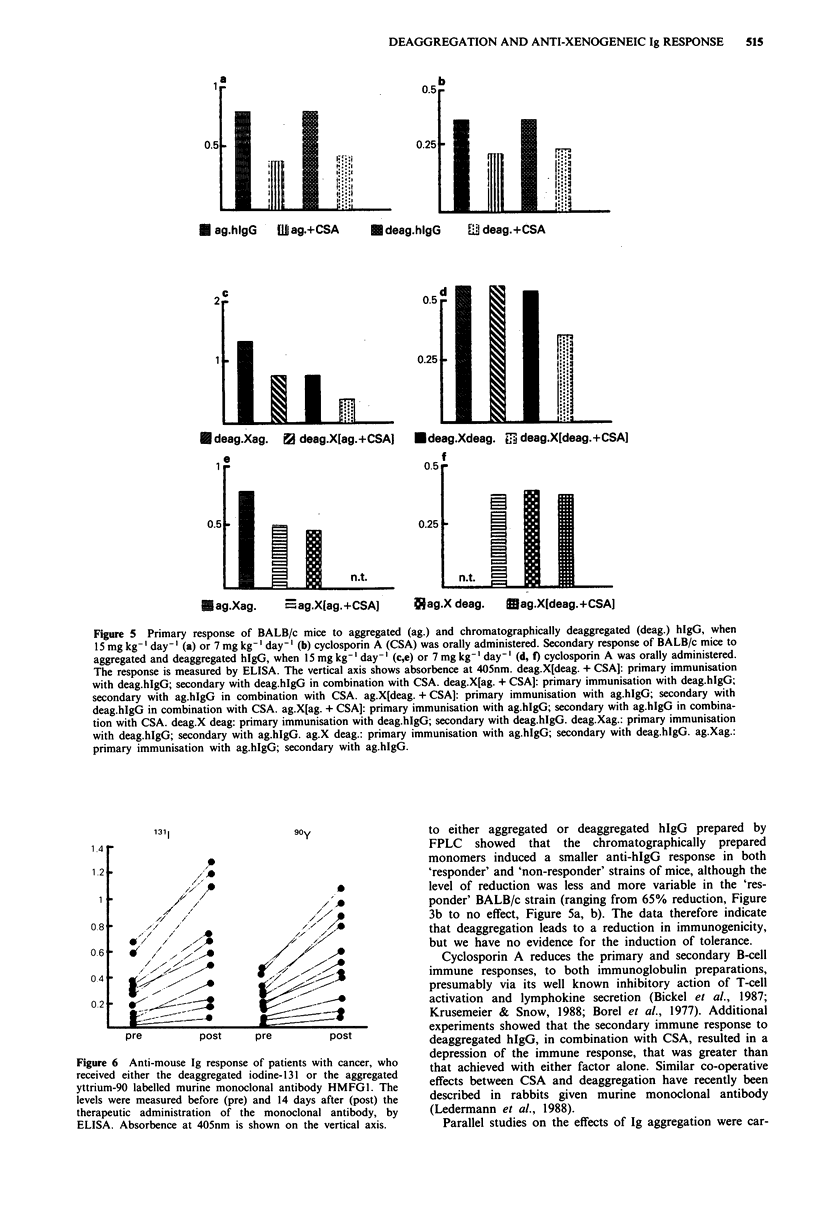
A major complication of in vivo monoclonal antibody therapy in patients with cancer is the host's immune response to the administered xenogeneic immunoglobulin. We have performed parallel clinical and experimental studies to investigate the possibility that deaggregation of the therapeutic monoclonal antibody might render it non-immunogenic, or even tolerogenic, as has been suggested in several animal studies. Deaggregation of xenogeneic immunoglobulin has been shown by others to induce non-responsiveness in some ('susceptible') but not in other ('resistant') strains of mice. We have used an improved deaggregation method of size exclusion chromatography connected to FPLC and have developed a sensitive ELISA detection system to determine whether highly purified human immunoglobulin G (hIgG) monomers could be tolerogenic even to 'resistant' mice. However, our data show that all preparations of hIgG are immunogenic to 'resistant' mice, and that although deaggregation does significantly reduce the anti-hIgG response to 'susceptible' strains, tolerance is not induced. Concomitant administration of cyclosporin A and deaggregated hIgG had a additive effect in reducing the murine anti-hIgG secondary response. In clinical studies of patients with ovarian cancer who received in vivo immunotherapy with either iodine-131 (not aggregated) or yttrium-90 (aggregated) HMFG1 mouse monoclonal antibody, no significant difference was found between the immune responses to aggregated and non-aggregated murine immunoglobulin G. Our data suggest that deaggregation alone is unlikely to be useful in controlling the human anti-murine immunoglobulin G response in our outbred patient population, although in combination with an immunosuppressant it may be more effective.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjamin R. J., Cobbold S. P., Clark M. R., Waldmann H. Tolerance to rat monoclonal antibodies. Implications for serotherapy. J Exp Med. 1986 Jun 1;163(6):1539–1552. doi: 10.1084/jem.163.6.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel M., Tsuda H., Amstad P., Evequoz V., Mergenhagen S. E., Wahl S. M., Pluznik D. H. Differential regulation of colony-stimulating factors and interleukin 2 production by cyclosporin A. Proc Natl Acad Sci U S A. 1987 May;84(10):3274–3277. doi: 10.1073/pnas.84.10.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel J. F., Feurer C., Magnée C., Stähelin H. Effects of the new anti-lymphocytic peptide cyclosporin A in animals. Immunology. 1977 Jun;32(6):1017–1025. [PMC free article] [PubMed] [Google Scholar]
- Carrasquillo J. A., Bunn P. A., Jr, Keenan A. M., Reynolds J. C., Schroff R. W., Foon K. A., Su M. H., Gazdar A. F., Mulshine J. L., Oldham R. K. Radioimmunodetection of cutaneous T-cell lymphoma with 111In-labeled T101 monoclonal antibody. N Engl J Med. 1986 Sep 11;315(11):673–680. doi: 10.1056/NEJM198609113151104. [DOI] [PubMed] [Google Scholar]
- Chatal J. F., Saccavini J. C., Fumoleau P., Douillard J. Y., Curtet C., Kremer M., Le Mevel B., Koprowski H. Immunoscintigraphy of colon carcinoma. J Nucl Med. 1984 Mar;25(3):307–314. [PubMed] [Google Scholar]
- Courtenay-Luck N. S., Epenetos A. A., Moore R., Larche M., Pectasides D., Dhokia B., Ritter M. A. Development of primary and secondary immune responses to mouse monoclonal antibodies used in the diagnosis and therapy of malignant neoplasms. Cancer Res. 1986 Dec;46(12 Pt 1):6489–6493. [PubMed] [Google Scholar]
- DRESSER D. W. Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology. 1962 May;5:378–388. [PMC free article] [PubMed] [Google Scholar]
- Das S., Leskowitz S. The kinetics of in vivo tolerance introduction in mice. J Immunol. 1970 Oct;105(4):938–943. [PubMed] [Google Scholar]
- Epenetos A. A., Munro A. J., Stewart S., Rampling R., Lambert H. E., McKenzie C. G., Soutter P., Rahemtulla A., Hooker G., Sivolapenko G. B. Antibody-guided irradiation of advanced ovarian cancer with intraperitoneally administered radiolabeled monoclonal antibodies. J Clin Oncol. 1987 Dec;5(12):1890–1899. doi: 10.1200/JCO.1987.5.12.1890. [DOI] [PubMed] [Google Scholar]
- Fujiwara M., Cinader B. Cellular aspects of tolerance. III. The responsiveness of T cells from tolerant donors after exposure to a cross-reacting antigen. Cell Immunol. 1974 Apr;12(1):1–10. doi: 10.1016/0008-8749(74)90051-3. [DOI] [PubMed] [Google Scholar]
- Golub E. S., Weigle W. O. Studies on the induction of immunologic unresponsiveness. 3. Antigen form and mouse strain variation. J Immunol. 1969 Feb;102(2):389–396. [PubMed] [Google Scholar]
- Hnatowich D. J., Childs R. L., Lanteigne D., Najafi A. The preparation of DTPA-coupled antibodies radiolabeled with metallic radionuclides: an improved method. J Immunol Methods. 1983 Dec 16;65(1-2):147–157. doi: 10.1016/0022-1759(83)90311-3. [DOI] [PubMed] [Google Scholar]
- Hosono M., Fujiwara M. Studies on the resistance to tolerance induction against human IgG in DDD mice. I. Organ differences of tolerogen susceptibility and cellular sites responsible for the resistance. Cell Immunol. 1979 Feb;42(2):279–288. doi: 10.1016/0008-8749(79)90193-x. [DOI] [PubMed] [Google Scholar]
- Hosono M., Fujiwara M. Studies on the resistance to tolerance induction against human IgG in DDD mice. II. Tolerogen-resistant T-cell population in the spleen. Immunology. 1979 Jun;37(2):353–359. [PMC free article] [PubMed] [Google Scholar]
- Krusemeier M., Snow E. C. Induction of lymphokine responsiveness of hapten-specific B lymphocytes promoted through an antigen-mediated T helper lymphocyte interaction. J Immunol. 1988 Jan 15;140(2):367–375. [PubMed] [Google Scholar]
- Ledermann J. A., Begent R. H., Bagshawe K. D. Cyclosporin A prevents the anti-murine antibody response to a monoclonal anti-tumour antibody in rabbits. Br J Cancer. 1988 Nov;58(5):562–566. doi: 10.1038/bjc.1988.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukic M. L., Leskowitz S. Tolerance induction with bovine gamma globulin in mouse radiation chimaeras depends on macrophages. Nature. 1974 Dec 13;252(5484):605–607. doi: 10.1038/252605a0. [DOI] [PubMed] [Google Scholar]
- Lukić M. L., Cowing C., Leskowitz S. Strain differences in ease of tolerance induction to bovine gamma-globulin: dependence on macrophage function. J Immunol. 1975 Jan;114(1 Pt 2):503–506. [PubMed] [Google Scholar]
- Lukić M. L., Wortis H. H., Leskowitz S. A gene locus affecting tolerance to BGG in mice. Cell Immunol. 1975 Feb;15(2):457–463. doi: 10.1016/0008-8749(75)90022-2. [DOI] [PubMed] [Google Scholar]
- Paik C. H., Hong J. J., Ebbert M. A., Heald S. C., Reba R. C., Eckelman W. C. Relative reactivity of DTPA, immunoreactive antibody-DTPA conjugates, and nonimmunoreactive antibody-DTPA conjugates toward indium-111. J Nucl Med. 1985 May;26(5):482–487. [PubMed] [Google Scholar]
- Pepys M. B., Taussig M. J. Complement-independence of tolerance induction. Eur J Immunol. 1974 May;4(5):349–352. doi: 10.1002/eji.1830040508. [DOI] [PubMed] [Google Scholar]
- Playfair J. H. Strain differences in the immune responses of mice. 3. A raised tolerance threshold in NZB thymus cells. Immunology. 1971 Dec;21(6):1037–1043. [PMC free article] [PubMed] [Google Scholar]
- Reay P. Use of N-bromosuccinimide for the iodination of proteins for radioimmunoassay. Ann Clin Biochem. 1982 Mar;19(Pt 2):129–133. doi: 10.1177/000456328201900214. [DOI] [PubMed] [Google Scholar]
- Schroff R. W., Foon K. A., Beatty S. M., Oldham R. K., Morgan A. C., Jr Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res. 1985 Feb;45(2):879–885. [PubMed] [Google Scholar]
- Staples P. J., Steinberg A. D., Talal N. Induction of immunologic tolerance in older New Zealand mice repopulated with young spleen, bone marrow, or thymus. J Exp Med. 1970 Jun 1;131(6):1223–1238. doi: 10.1084/jem.131.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Peterson J. A., Arklie J., Burchell J., Ceriani R. L., Bodmer W. F. Monoclonal antibodies to epithelium-specific components of the human milk fat globule membrane: production and reaction with cells in culture. Int J Cancer. 1981 Jul 15;28(1):17–21. doi: 10.1002/ijc.2910280104. [DOI] [PubMed] [Google Scholar]
- Weigle W. O. Immunological unresponsiveness. Adv Immunol. 1973;16:61–122. doi: 10.1016/s0065-2776(08)60296-5. [DOI] [PubMed] [Google Scholar]


