Abstract
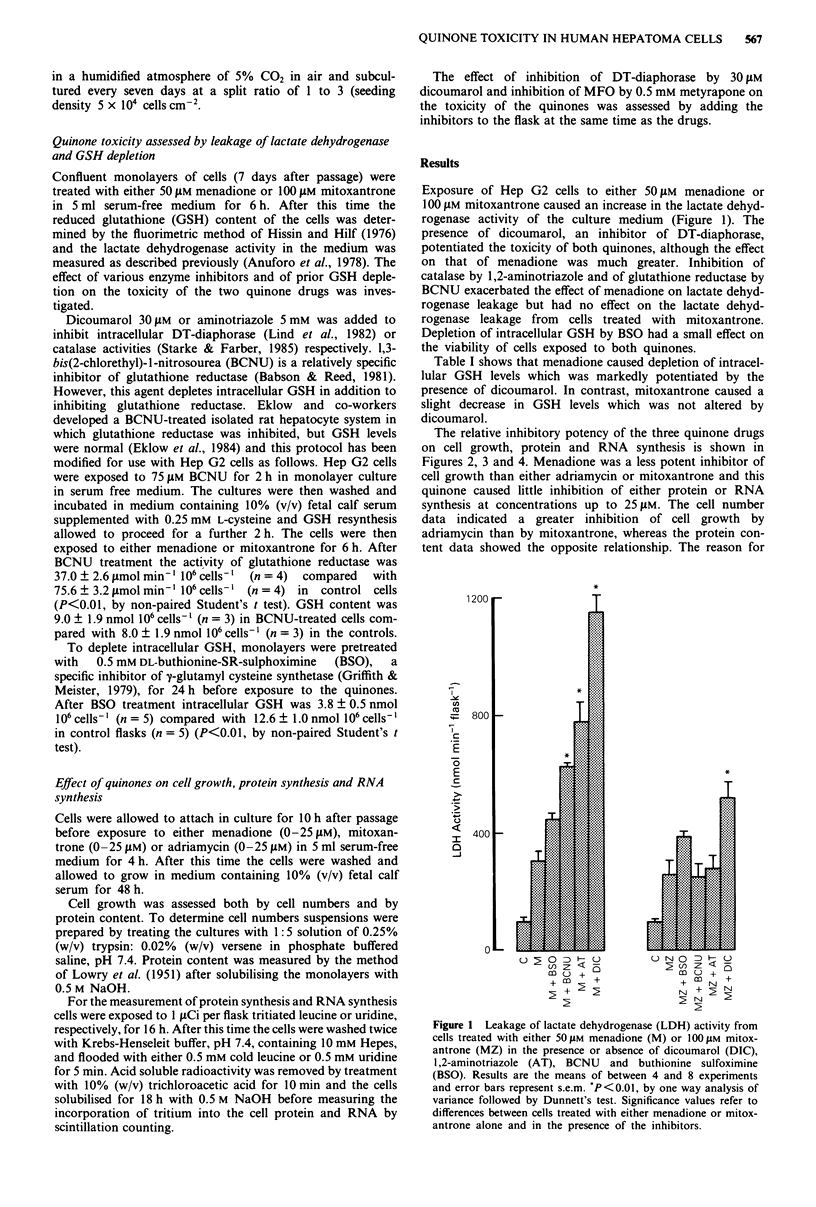
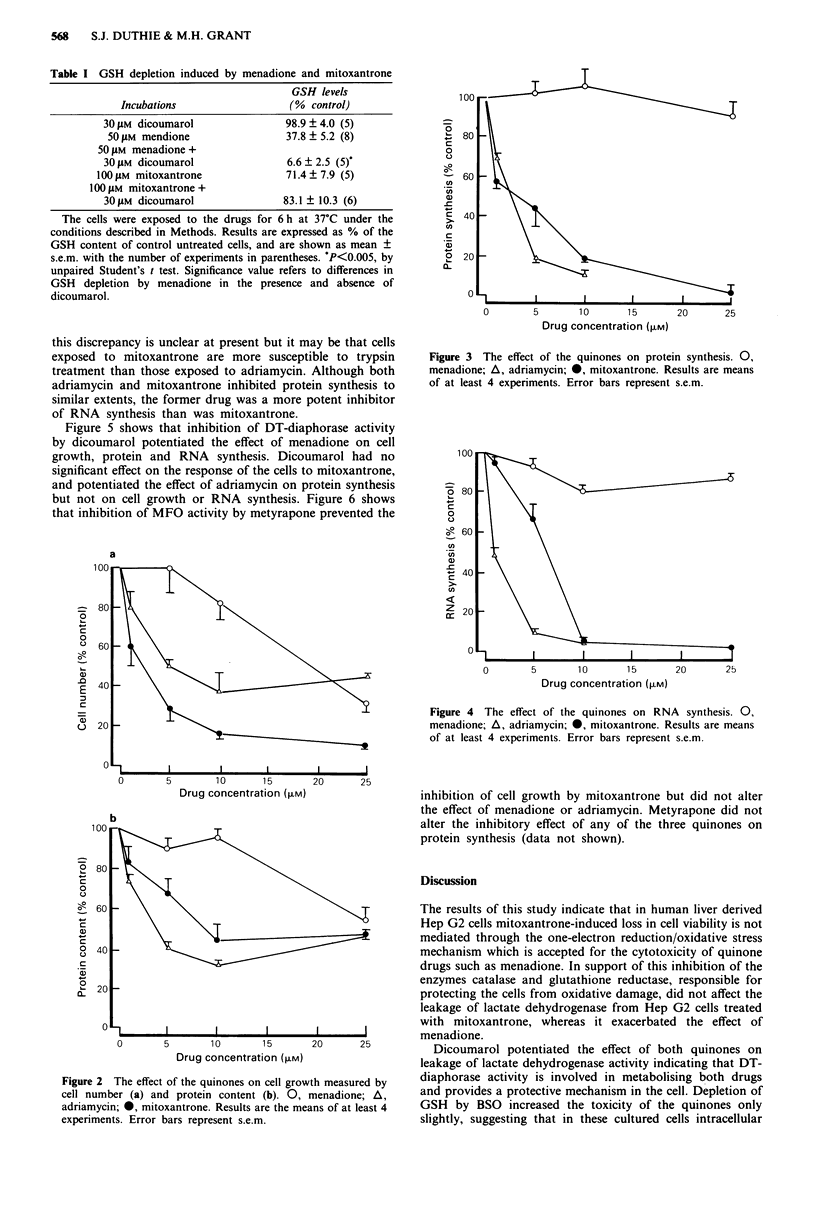
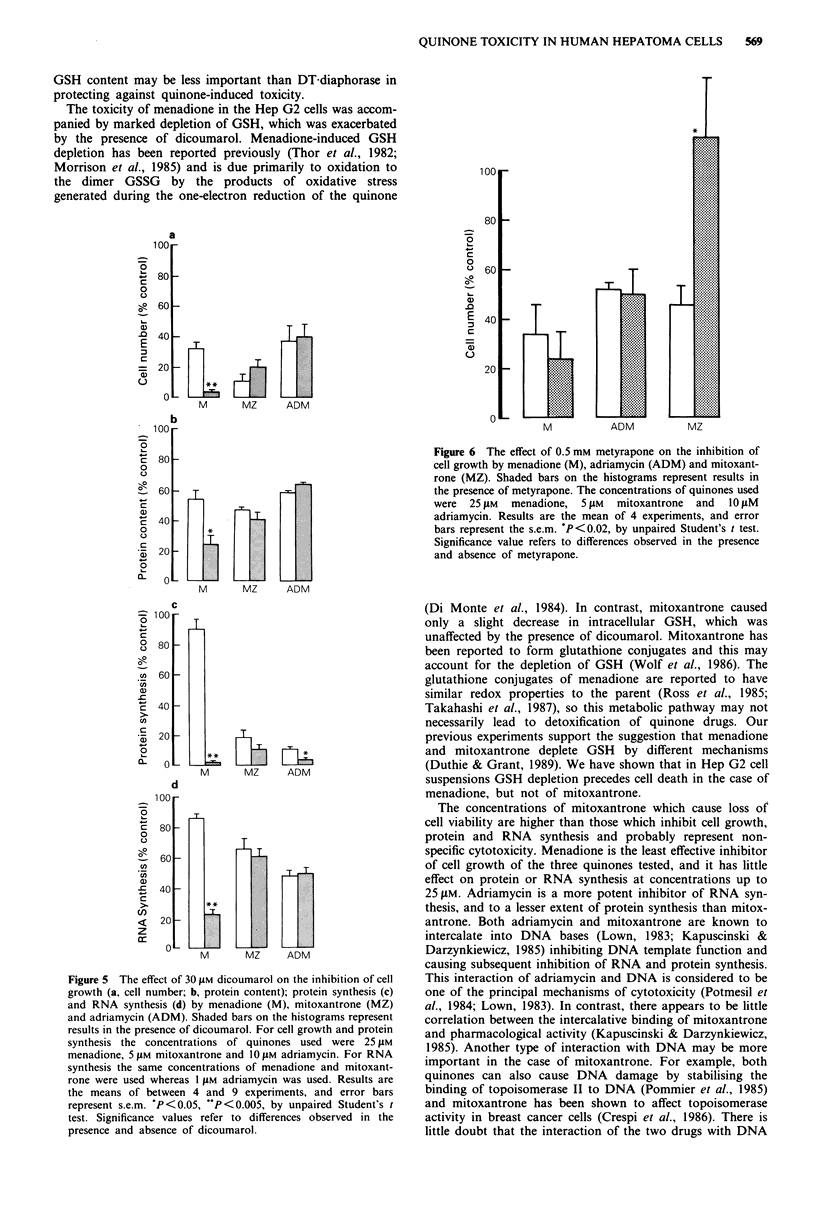
The cytotoxic properties of quinones, such as menadione, are mediated through one electron reduction to yield semi-quinone radicals which can subsequently enter redox cycles with molecular oxygen leading to the formation of reactive oxygen radicals. In this study the role of reduction and oxidation in the toxicity of mitoxantrone was studied and its toxicity compared with that of adriamycin and menadione. The acute toxicity of mitoxantrone was not mediated through one-electron reduction, since inhibition of the enzymes glutathione reductase and catalase, responsible for protecting the cells against oxidative damage, did not affect its toxicity. Adriamycin was the most potent inhibitor of protein and RNA synthesis of the three quinones. Menadione, at concentrations up to 25 microM, did not inhibit either protein or RNA synthesis unless dicoumarol, an inhibitor of DT-diaphorase, was also present. The two-electron reduction of menadione by DT-diaphorase is therefore a protective mechanism in the cell. This enzyme also protected against the toxicity of high concentrations (100 microM) of mitoxantrone. The inhibitory effect of mitoxantrone, but not of menadione or adriamycin, on cell growth was prevented by inhibiting the activity of cytochrome P450-dependent mixed function oxidase (MFO) system using metyrapone. This suggests that mitoxantrone is oxidised to a toxic intermediate by the MFO system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anuforo D. C., Acosta D., Smith R. V. Hepatotoxicity studies with primary cultures of rat liver cells. In Vitro. 1978 Dec;14(12):981–988. doi: 10.1007/BF02616211. [DOI] [PubMed] [Google Scholar]
- Arcamone F. Antitumor anthracyclines: recent developments. Med Res Rev. 1984 Apr-Jun;4(2):153–188. doi: 10.1002/med.2610040203. [DOI] [PubMed] [Google Scholar]
- Babson J. R., Abell N. S., Reed D. J. Protective role of the glutathione redox cycle against adriamycin-mediated toxicity in isolated hepatocytes. Biochem Pharmacol. 1981 Aug 15;30(16):2299–2304. doi: 10.1016/0006-2952(81)90102-7. [DOI] [PubMed] [Google Scholar]
- Begleiter A., Grover J., Froese E., Goldenberg G. J. Membrane transport, sulfhydryl levels and DNA cross-linking in Chinese hamster ovary cell mutants sensitive and resistant to melphalan. Biochem Pharmacol. 1983 Jan 15;32(2):293–300. doi: 10.1016/0006-2952(83)90558-0. [DOI] [PubMed] [Google Scholar]
- Butler J., Hoey B. M. Are reduced quinones necessarily involved in the antitumour activity of quinone drugs? Br J Cancer Suppl. 1987 Jun;8:53–59. [PMC free article] [PubMed] [Google Scholar]
- Chesis P. L., Levin D. E., Smith M. T., Ernster L., Ames B. N. Mutagenicity of quinones: pathways of metabolic activation and detoxification. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1696–1700. doi: 10.1073/pnas.81.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornbleet M. A., Stuart-Harris R. C., Smith I. E., Coleman R. E., Rubens R. D., McDonald M., Mouridsen H. T., Rainer H., van Oosterom A. T., Smyth J. F. Mitoxantrone for the treatment of advanced breast cancer: single-agent therapy in previously untreated patients. Eur J Cancer Clin Oncol. 1984 Sep;20(9):1141–1146. doi: 10.1016/0277-5379(84)90122-6. [DOI] [PubMed] [Google Scholar]
- Crespi M. D., Ivanier S. E., Genovese J., Baldi A. Mitoxantrone affects topoisomerase activities in human breast cancer cells. Biochem Biophys Res Commun. 1986 Apr 29;136(2):521–528. doi: 10.1016/0006-291x(86)90471-7. [DOI] [PubMed] [Google Scholar]
- Di Monte D., Bellomo G., Thor H., Nicotera P., Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch Biochem Biophys. 1984 Dec;235(2):343–350. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- Duthie S. J., Grant M. H. The toxicity of menadione and mitozantrone in human liver-derived Hep G2 hepatoma cells. Biochem Pharmacol. 1989 Apr 15;38(8):1247–1255. doi: 10.1016/0006-2952(89)90330-4. [DOI] [PubMed] [Google Scholar]
- Eklöw L., Moldéus P., Orrenius S. Oxidation of glutathione during hydroperoxide metabolism. A study using isolated hepatocytes and the glutathione reductase inhibitor 1,3-bis(2-chloroethyl)-1-nitrosourea. Eur J Biochem. 1984 Feb 1;138(3):459–463. doi: 10.1111/j.1432-1033.1984.tb07938.x. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissin P. J., Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976 Jul;74(1):214–226. doi: 10.1016/0003-2697(76)90326-2. [DOI] [PubMed] [Google Scholar]
- Iyanagi T., Yamazaki I. One-electron-transfer reactions in biochemical systems. V. Difference in the mechanism of quinone reduction by the NADH dehydrogenase and the NAD(P)H dehydrogenase (DT-diaphorase). Biochim Biophys Acta. 1970 Sep 1;216(2):282–294. doi: 10.1016/0005-2728(70)90220-3. [DOI] [PubMed] [Google Scholar]
- Kappus H. Overview of enzyme systems involved in bio-reduction of drugs and in redox cycling. Biochem Pharmacol. 1986 Jan 1;35(1):1–6. doi: 10.1016/0006-2952(86)90544-7. [DOI] [PubMed] [Google Scholar]
- Kapuscinski J., Darzynkiewicz Z. Interactions of antitumor agents Ametantrone and Mitoxantrone (Novatrone) with double-stranded DNA. Biochem Pharmacol. 1985 Dec 15;34(24):4203–4213. doi: 10.1016/0006-2952(85)90275-8. [DOI] [PubMed] [Google Scholar]
- Kharasch E. D., Novak R. F. Anthracenedione activation by NADPH-cytochrome P-450 reductase; comparison with anthracyclines. Biochem Pharmacol. 1981 Oct;30(20):2881–2884. doi: 10.1016/0006-2952(81)90433-0. [DOI] [PubMed] [Google Scholar]
- Kharasch E. D., Novak R. F. Bis(alkylamino)anthracenedione antineoplastic agent metabolic activation by NADPH-cytochrome P-450 reductase and NADH dehydrogenase: diminished activity relative to anthracyclines. Arch Biochem Biophys. 1983 Jul 15;224(2):682–694. doi: 10.1016/0003-9861(83)90256-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lind C., Hochstein P., Ernster L. DT-diaphorase as a quinone reductase: a cellular control device against semiquinone and superoxide radical formation. Arch Biochem Biophys. 1982 Jun;216(1):178–185. doi: 10.1016/0003-9861(82)90202-8. [DOI] [PubMed] [Google Scholar]
- Lown J. W., Morgan A. R., Yen S. F., Wang Y. H., Wilson W. D. Characteristics of the binding of the anticancer agents mitoxantrone and ametantrone and related structures to deoxyribonucleic acids. Biochemistry. 1985 Jul 16;24(15):4028–4035. doi: 10.1021/bi00336a034. [DOI] [PubMed] [Google Scholar]
- Lown J. W. The mechanism of action of quinone antibiotics. Mol Cell Biochem. 1983;55(1):17–40. doi: 10.1007/BF00229240. [DOI] [PubMed] [Google Scholar]
- Mimnaugh E. G., Gram T. E., Trush M. A. Stimulation of mouse heart and liver microsomal lipid peroxidation by anthracycline anticancer drugs: characterization and effects of reactive oxygen scavengers. J Pharmacol Exp Ther. 1983 Sep;226(3):806–816. [PubMed] [Google Scholar]
- Mimnaugh E. G., Trush M. A., Ginsburg E., Gram T. E. Differential effects of anthracycline drugs on rat heart and liver microsomal reduced nicotinamide adenine dinucleotide phosphate-dependent lipid peroxidation. Cancer Res. 1982 Sep;42(9):3574–3582. [PubMed] [Google Scholar]
- Morrison H., Di Monte D., Nordenskjöld M., Jernström B. Induction of cell damage by menadione and benzo(a)pyrene-3,6-quinone in cultures of adult rat hepatocytes and human fibroblasts. Toxicol Lett. 1985 Oct;28(1):37–47. doi: 10.1016/0378-4274(85)90007-4. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Schwartz R. E., Zwelling L. A., Kohn K. W. Effects of DNA intercalating agents on topoisomerase II induced DNA strand cleavage in isolated mammalian cell nuclei. Biochemistry. 1985 Nov 5;24(23):6406–6410. doi: 10.1021/bi00344a014. [DOI] [PubMed] [Google Scholar]
- Potmesil M., Israel M., Silber R. Two mechanisms of adriamycin-DNA interaction in L1210 cells. Biochem Pharmacol. 1984 Oct 15;33(20):3137–3142. doi: 10.1016/0006-2952(84)90069-8. [DOI] [PubMed] [Google Scholar]
- Reszka K., Kolodziejczyk P., Lown J. W. Horseradish peroxidase-catalyzed oxidation of mitoxantrone: spectrophotometric and electron paramagnetic resonance studies. J Free Radic Biol Med. 1986;2(1):25–32. doi: 10.1016/0748-5514(86)90120-0. [DOI] [PubMed] [Google Scholar]
- Ross D., Thor H., Orrenius S., Moldeus P. Interaction of menadione (2-methyl-1,4-naphthoquinone) with glutathione. Chem Biol Interact. 1985 Oct;55(1-2):177–184. doi: 10.1016/s0009-2797(85)80126-5. [DOI] [PubMed] [Google Scholar]
- Smith I. E. Mitoxantrone (novantrone): a review of experimental and early clinical studies. Cancer Treat Rev. 1983 Jun;10(2):103–115. doi: 10.1016/0305-7372(83)90008-7. [DOI] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Endogenous defenses against the cytotoxicity of hydrogen peroxide in cultured rat hepatocytes. J Biol Chem. 1985 Jan 10;260(1):86–92. [PubMed] [Google Scholar]
- Takahashi N., Schreiber J., Fischer V., Mason R. P. Formation of glutathione-conjugated semiquinones by the reaction of quinones with glutathione: an ESR study. Arch Biochem Biophys. 1987 Jan;252(1):41–48. doi: 10.1016/0003-9861(87)90006-3. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Wolf C. R., Macpherson J. S., Smyth J. F. Evidence for the metabolism of mitozantrone by microsomal glutathione transferases and 3-methylcholanthrene-inducible glucuronosyl transferases. Biochem Pharmacol. 1986 May 1;35(9):1577–1581. doi: 10.1016/0006-2952(86)90127-9. [DOI] [PubMed] [Google Scholar]


