Abstract
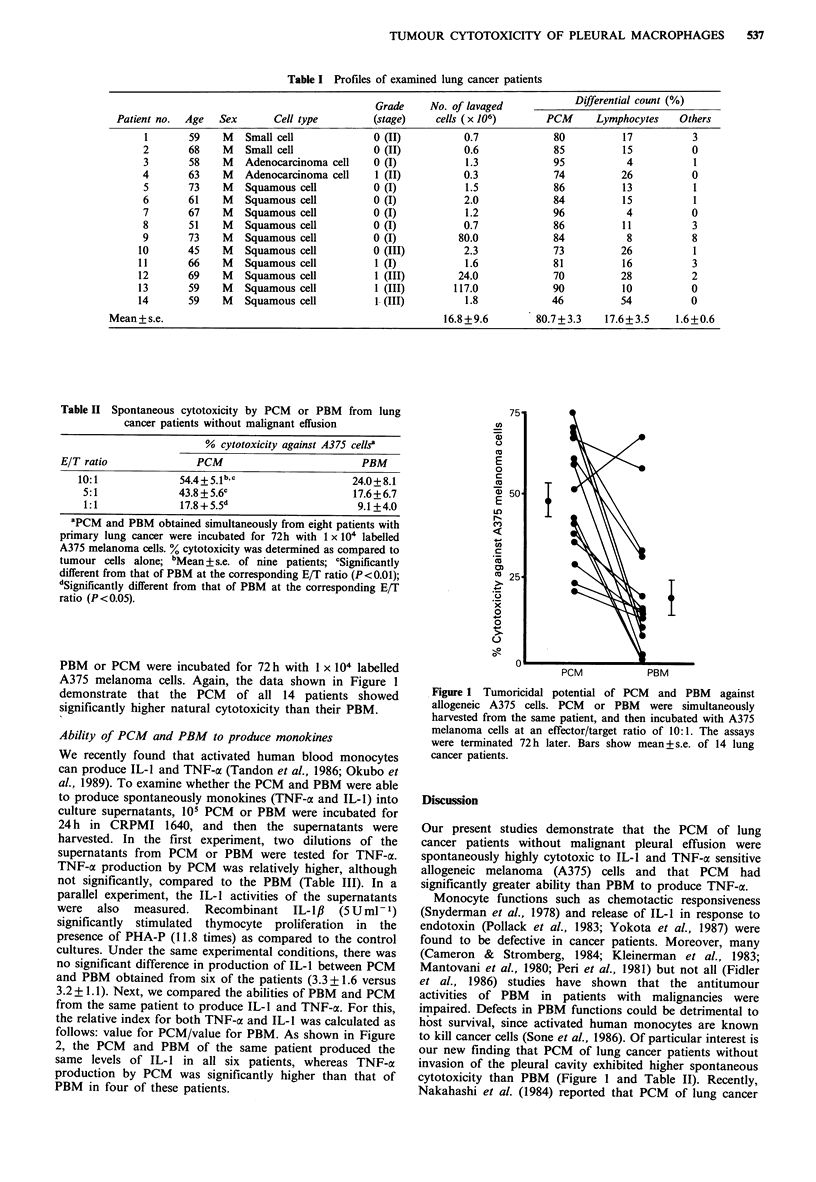
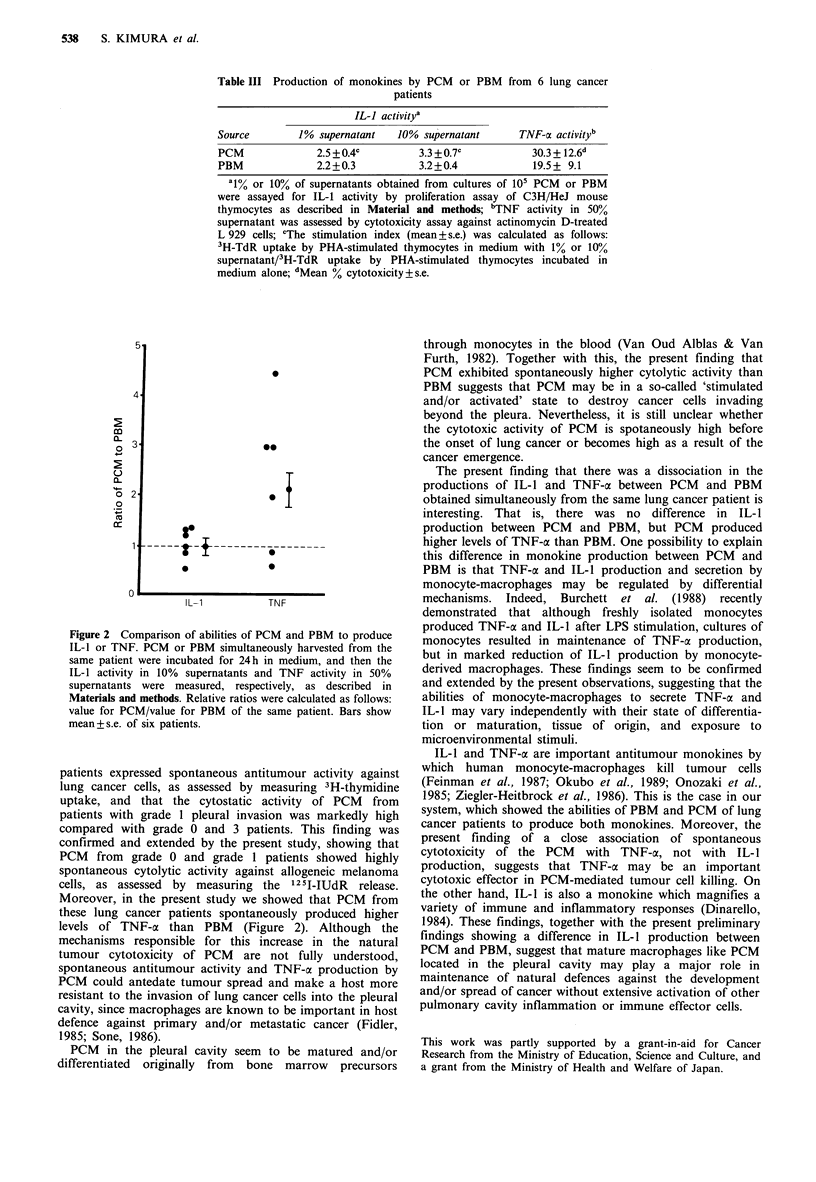
The present study was undertaken to examine whether the presence of primary lung cancer could affect the antitumour activities of pleural cavity macrophages (PCM) and peripheral blood monocytes (PBM). PCM by pleural lavage and PBM were simultaneously obtained from 14 lung cancer patients not showing invasion of the pleural cavity. PCM and PBM were isolated by percoll gradient centrifugation and adherence. The lavage method yielded about 16.8 +/- 9.6 (s.e.) x 10(6) cells, which consisted of 80.7% PCM, 17.6% lymphocytes and 1.6% other cells. The cytotoxic activities of PCM and PBM against allogeneic melanoma (A375) cells were assessed by a 72h 125I-IUdR release assay. The lavaged PCM showed spontaneously high tumour cytotoxic activity which was dependent on the effector/target ratio. In 13 out of 14 cancer patients, PCM were significantly more cytotoxic to melanoma cells than PBM. In contrast, there were no significant differences in production of tumour necrosis factor (TNF-alpha) or interleukin 1 (IL-1) between PCM and PBM. When the abilities of PCM and PBM of the same patient to produce these monokines were compared, PCM produced much more TNF-alpha than PBM, thus indicating a correlation between the expression of spontaneous macrophage-mediated cytotoxicity and spontaneous TNF-alpha production by PCM. These results suggest that PCM may play an important role in host defence against invasion of the pleural cavity by cancer cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blussé van Oud Alblas A., van Furth R. The origin of pulmonary macrophages. Immunobiology. 1982 Apr;161(3-4):186–192. doi: 10.1016/S0171-2985(82)80073-9. [DOI] [PubMed] [Google Scholar]
- Burchett S. K., Weaver W. M., Westall J. A., Larsen A., Kronheim S., Wilson C. B. Regulation of tumor necrosis factor/cachectin and IL-1 secretion in human mononuclear phagocytes. J Immunol. 1988 May 15;140(10):3473–3481. [PubMed] [Google Scholar]
- Cameron D. J., Stromberg B. V. The ability of macrophages from head and neck cancer patients to kill tumor cells. Effect of prostaglandin inhibitors on cytotoxicity. Cancer. 1984 Dec 1;54(11):2403–2408. doi: 10.1002/1097-0142(19841201)54:11<2403::aid-cncr2820541116>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Colotta F., Peri G., Villa A., Mantovani A. Rapid killing of actinomycin D-treated tumor cells by human mononuclear cells. I. Effectors belong to the monocyte-macrophage lineage. J Immunol. 1984 Feb;132(2):936–944. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Feinman R., Henriksen-DeStefano D., Tsujimoto M., Vilcek J. Tumor necrosis factor is an important mediator of tumor cell killing by human monocytes. J Immunol. 1987 Jan 15;138(2):635–640. [PubMed] [Google Scholar]
- Fidler I. J., Jessup J. M., Fogler W. E., Staerkel R., Mazumder A. Activation of tumoricidal properties in peripheral blood monocytes of patients with colorectal carcinoma. Cancer Res. 1986 Feb;46(2):994–998. [PubMed] [Google Scholar]
- Fidler I. J. Macrophages and metastasis--a biological approach to cancer therapy. Cancer Res. 1985 Oct;45(10):4714–4726. [PubMed] [Google Scholar]
- Gauci C. L. The significance of the macrophage content of human tumours. Recent Results Cancer Res. 1976;(56):122–130. doi: 10.1007/978-3-642-81049-7_16. [DOI] [PubMed] [Google Scholar]
- Gudewicz P. W., Saba T. M. Inhibition of phagocytosis and glucose metabolism of alveolar macrophages during pulmonary tumour growth. Br J Cancer. 1977 Dec;36(6):670–677. doi: 10.1038/bjc.1977.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausheer F. H., Yarbro J. W. Diagnosis and treatment of malignant pleural effusion. Semin Oncol. 1985 Mar;12(1):54–75. [PubMed] [Google Scholar]
- Kleinerman E. S., Erickson K. L., Schroit A. J., Fogler W. E., Fidler I. J. Activation of tumoricidal properties in human blood monocytes by liposomes containing lipophilic muramyl tripeptide. Cancer Res. 1983 May;43(5):2010–2014. [PubMed] [Google Scholar]
- Lauder I., Aherne W., Stewart J., Sainsbury R. Macrophage infiltration of breast tumours: a prospective study. J Clin Pathol. 1977 Jun;30(6):563–568. doi: 10.1136/jcp.30.6.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemarbre P., Hoidal J., Vesella R., Rinehart J. Human pulmonary macrophage tumor cell cytotoxicity. Blood. 1980 Apr;55(4):612–617. [PubMed] [Google Scholar]
- Mantovani A., Polentarutti N., Peri G., Shavit Z. B., Vecchi A., Bolis G., Mangioni C. Cytotoxicity on tumor cells of peripheral blood monocytes and tumor-associated macrophages in patients with ascites ovarian tumors. J Natl Cancer Inst. 1980 Jun;64(6):1307–1315. doi: 10.1093/jnci/64.6.1307. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Stevenson M. M. Macrophage function in tumor-bearing mice: dissociation of phagocytic and chemotactic responsiveness. Cell Immunol. 1978 Jan;35(1):99–111. doi: 10.1016/0008-8749(78)90130-2. [DOI] [PubMed] [Google Scholar]
- Meltzer M. S., Stevenson M. M. Macrophage function in tumor-bearing mice: tumoricidal and chemotactic responses of macrophages activated by infection with Mycobacterium bovis, strain BCG. J Immunol. 1977 Jun;118(6):2176–2181. [PubMed] [Google Scholar]
- Nagashima A., Yasumoto K., Nakahashi H., Takeo S., Yano T., Nomoto K. Antitumor activity of pleural cavity macrophages and its regulation by pleural cavity lymphocytes in patients with lung cancer. Cancer Res. 1987 Oct 15;47(20):5497–5500. [PubMed] [Google Scholar]
- Nakahashi H., Yasumoto K., Nagashima A., Yaita H., Takeo S., Motohiro A., Furukawa T., Inokuchi K., Nomoto K. Antitumor activity of macrophages in lung cancer patients with special reference to location of macrophages. Cancer Res. 1984 Dec;44(12 Pt 1):5906–5909. [PubMed] [Google Scholar]
- Nishida T., Nishino N., Takano M., Kawai K., Bando K., Masui Y., Nakai S., Hirai Y. cDNA cloning of IL-1 alpha and IL-1 beta from mRNA of U937 cell line. Biochem Biophys Res Commun. 1987 Feb 27;143(1):345–352. doi: 10.1016/0006-291x(87)90671-1. [DOI] [PubMed] [Google Scholar]
- Okubo A., Sone S., Tanaka M., Ogura T. Membrane-associated interleukin 1 alpha as a mediator of tumor cell killing by human blood monocytes fixed with paraformaldehyde. Cancer Res. 1989 Jan 15;49(2):265–270. [PubMed] [Google Scholar]
- Onozaki K., Matsushima K., Aggarwal B. B., Oppenheim J. J. Human interleukin 1 is a cytocidal factor for several tumor cell lines. J Immunol. 1985 Dec;135(6):3962–3968. [PubMed] [Google Scholar]
- Pasternack G. R., Snyderman R., Pike M. C., Johnson R. J., Shin H. S. Resistance of neoplasms to immunological destruction: role of a macrophage chemotaxis inhibitor. J Exp Med. 1978 Jul 1;148(1):93–102. doi: 10.1084/jem.148.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri G., Polentarutti N., Sessa C., Mangioni C., Mantovani A. Tumoricidal activity of macrophages isolated from human ascitic and solid ovarian carcinomas: augmentation by interferon, lymphokines and endotoxin. Int J Cancer. 1981 Aug 15;28(2):143–152. doi: 10.1002/ijc.2910280206. [DOI] [PubMed] [Google Scholar]
- Pollack S., Micali A., Kinne D. W., Enker W. E., Geller N., Oettgen H. F., Hoffmann M. K. Endotoxin-induced in vitro release of interleukin-1 by cancer patients' monocytes: relation to stage of disease. Int J Cancer. 1983 Dec 15;32(6):733–736. doi: 10.1002/ijc.2910320613. [DOI] [PubMed] [Google Scholar]
- Rhodes J. Altered expression of human monocyte Fc receptors in malignant disease. Nature. 1977 Jan 20;265(5591):253–255. doi: 10.1038/265253a0. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Meadows L., Holder W., Wells S., Jr Abnormal monocyte chemotaxis in patients with breast cancer: evidence for a tumor-mediated effect. J Natl Cancer Inst. 1978 Apr;60(4):737–740. doi: 10.1093/jnci/60.4.737. [DOI] [PubMed] [Google Scholar]
- Sone S., Fidler I. J. Activation of rat alveolar macrophages to the tumoricidal state in the presence of progressively growing pulmonary metastases. Cancer Res. 1981 Jun;41(6):2401–2406. [PubMed] [Google Scholar]
- Sone S., Moriguchi S., Shimizu E., Ogushi F., Tsubura E. In vitro generation of tumoricidal properties in human alveolar macrophages following interaction with endotoxin. Cancer Res. 1982 Jun;42(6):2227–2231. [PubMed] [Google Scholar]
- Sone S. Role of alveolar macrophages in pulmonary neoplasias. Biochim Biophys Acta. 1986;823(3):227–245. doi: 10.1016/0304-419x(86)90004-1. [DOI] [PubMed] [Google Scholar]
- Sone S., Tachibana K., Ishii K., Ogawara M., Tsubura E. Production of a tumor cytolytic factor(s) by activated human alveolar macrophages and its action. Cancer Res. 1984 Feb;44(2):646–651. [PubMed] [Google Scholar]
- Sone S., Tandon P., Utsugi T., Ogawara M., Shimizu E., Nii A., Ogura T. Synergism of recombinant human interferon gamma with liposome-encapsulated muramyl tripeptide in activation of the tumoricidal properties of human monocytes. Int J Cancer. 1986 Oct 15;38(4):495–500. doi: 10.1002/ijc.2910380407. [DOI] [PubMed] [Google Scholar]
- Sone S., Tsubura E. Human alveolar macrophages: potentiation of their tumoricidal activity by liposome-encapsulated muramyl dipeptide. J Immunol. 1982 Sep;129(3):1313–1317. [PubMed] [Google Scholar]
- Tandon P., Utsugi T., Sone S. Lack of production of interleukin 1 by human blood monocytes activated to the antitumor state by liposome-encapsulated muramyl tripeptide. Cancer Res. 1986 Oct;46(10):5039–5044. [PubMed] [Google Scholar]
- Utsugi T., Sone S. Comparative analysis of the priming effect of human interferon-gamma, -alpha, and -beta on synergism with muramyl dipeptide analog for anti-tumor expression of human blood monocytes. J Immunol. 1986 Feb 1;136(3):1117–1122. [PubMed] [Google Scholar]
- Yokota M., Sakamoto S., Koga S., Ibayashi H. Decreased interleukin 1 activity in culture supernatant of lipopolysaccharide stimulated monocytes from patients with liver cirrhosis and hepatocellular carcinoma. Clin Exp Immunol. 1987 Feb;67(2):335–342. [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Möller A., Linke R. P., Haas J. G., Rieber E. P., Riethmüller G. Tumor necrosis factor as effector molecule in monocyte mediated cytotoxicity. Cancer Res. 1986 Nov;46(11):5947–5952. [PubMed] [Google Scholar]


