Full text
PDF

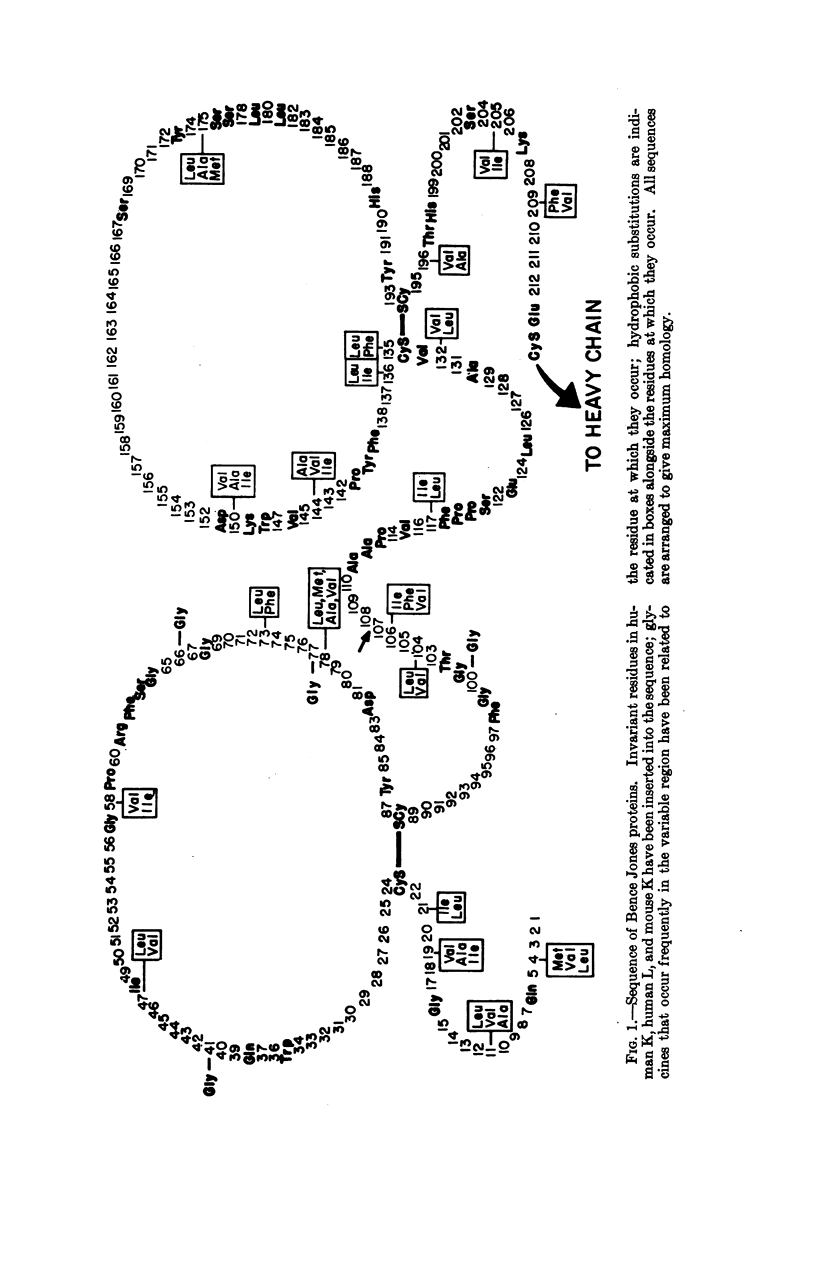




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gray W. R., Dreyer W. J., Hood L. Mechanism of antibody synthesis: size differences between mouse kappa chains. Science. 1967 Jan 27;155(3761):465–467. doi: 10.1126/science.155.3761.465. [DOI] [PubMed] [Google Scholar]
- Hilschmann N., Craig L. C. Amino acid sequence studies with Bence-Jones proteins. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1403–1409. doi: 10.1073/pnas.53.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilschmann N. Die chemische Struktur von zwei Bence-Jones-Proteinen (roy und Cum.) vom kappa-Typ. Hoppe Seylers Z Physiol Chem. 1967 Aug;348(8):1077–1080. [PubMed] [Google Scholar]
- Hood L. E., Gray W. R., Dreyer W. J. On the mechanism of antibody synthesis: a species comparison of L-chains. Proc Natl Acad Sci U S A. 1966 Apr;55(4):826–832. doi: 10.1073/pnas.55.4.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat E. A. The paucity of species-specific amino acid residues in the variable regions of human and mouse Bence-Jones proteins and its evolutionary and genetic implications. Proc Natl Acad Sci U S A. 1967 May;57(5):1345–1349. doi: 10.1073/pnas.57.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G. Ligand-binding sites for streptolysin O and staphylococcal protein A on different parts of the same myeloma globulin. Acta Pathol Microbiol Scand. 1967;69(4):619–621. doi: 10.1111/j.1699-0463.1967.tb03776.x. [DOI] [PubMed] [Google Scholar]
- Leach S. J., Némethy G., Scheraga H. A. Computation of the sterically allowed conformations of peptides. Biopolymers. 1966 Apr-May;4(4):369–407. doi: 10.1002/bip.1966.360040402. [DOI] [PubMed] [Google Scholar]
- Levinthal C. Molecular model-building by computer. Sci Am. 1966 Jun;214(6):42–52. doi: 10.1038/scientificamerican0666-42. [DOI] [PubMed] [Google Scholar]
- Metzger H. Characterization of a human macroglobulin. V. A Waldenström macroglobulin with antibody activity. Proc Natl Acad Sci U S A. 1967 May;57(5):1490–1497. doi: 10.1073/pnas.57.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C. Chemical structure of light chains. Proc R Soc Lond B Biol Sci. 1966 Nov 22;166(1003):138–149. doi: 10.1098/rspb.1966.0089. [DOI] [PubMed] [Google Scholar]
- Milstein C., Clegg J. B., Jarvis J. M. C-terminal half of immunoglobulin lambda-chains. Nature. 1967 Apr 15;214(5085):270–272. doi: 10.1038/214270a0. [DOI] [PubMed] [Google Scholar]
- Milstein C. Linked groups of residues in immunoglobulin k chains. Nature. 1967 Oct 28;216(5113):330–332. doi: 10.1038/216330a0. [DOI] [PubMed] [Google Scholar]
- Milstein C. Variations in amino-acid sequence near the disulphide bridges of Bence-Jones proteins. Nature. 1966 Jan 22;209(5021):370–373. doi: 10.1038/209370a0. [DOI] [PubMed] [Google Scholar]
- Niall H. D., Edman P. Two structurally distinct classes of kappa-chains in human immunoglobulins. Nature. 1967 Oct 21;216(5112):262–263. doi: 10.1038/216262a0. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Press E. M. Cyanogen bromide cleavage and partial sequence of the heavy chain of a pathological immunoglobulin G. Biochem J. 1967 Aug;104(2):616–626. doi: 10.1042/bj1040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam F. W., Shinoda T., Titani K., Wikler M. Immunoglobulin structure: variation in amino acid sequence and length of human lambda light chains. Science. 1967 Sep 1;157(3792):1050–1053. doi: 10.1126/science.157.3792.1050. [DOI] [PubMed] [Google Scholar]
- Putnam F. W., Titani K., Whitley E., Jr Chemical structure of light chains: amino acid sequence of type K Bence-Jones proteins. Proc R Soc Lond B Biol Sci. 1966 Nov 22;166(1003):124–137. doi: 10.1098/rspb.1966.0088. [DOI] [PubMed] [Google Scholar]
- Titani K., Whitley E., Jr, Avogardo L., Putnam F. W. Immunoglobulin structure: partial amino acid sequence of a Bence Jones protein. Science. 1965 Sep 3;149(3688):1090–1092. doi: 10.1126/science.149.3688.1090. [DOI] [PubMed] [Google Scholar]
- Titani K., Whitley E., Jr, Putnam F. W. Immunoglobulin structure: variation in the sequence of Bence Jones proteins. Science. 1966 Jun 10;152(3728):1513–1516. doi: 10.1126/science.152.3728.1513. [DOI] [PubMed] [Google Scholar]
- Titani K., Wikler M., Putnam F. W. Evolution of immunoglobulins: structural homology of kappa and lambda Bence Jones proteins. Science. 1967 Feb 17;155(3764):828–835. doi: 10.1126/science.155.3764.828. [DOI] [PubMed] [Google Scholar]
- Wikler M., Titani K., Shinoda T., Putnam F. W. The complete amino acid sequence of a lambda type Bence-Jones protein. J Biol Chem. 1967 Apr 10;242(7):1668–1670. [PubMed] [Google Scholar]



