Abstract
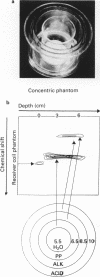
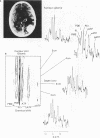
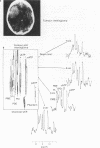
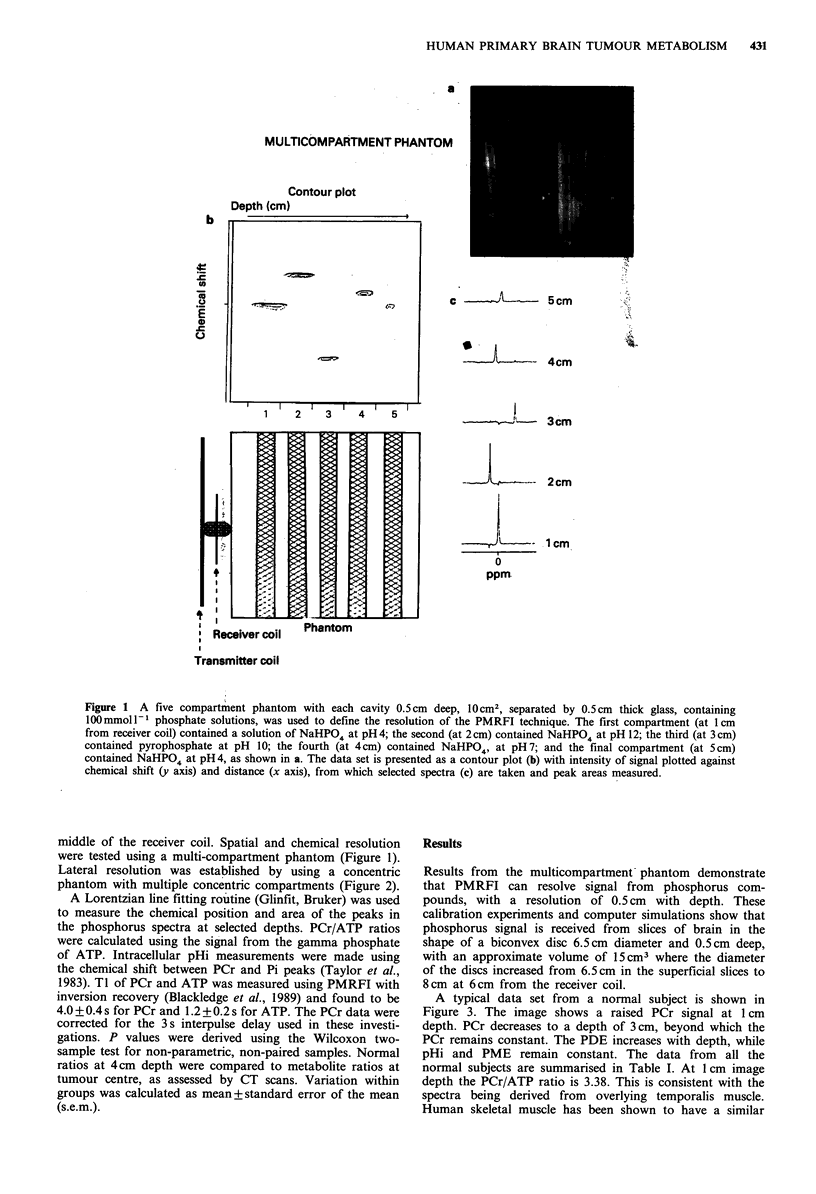
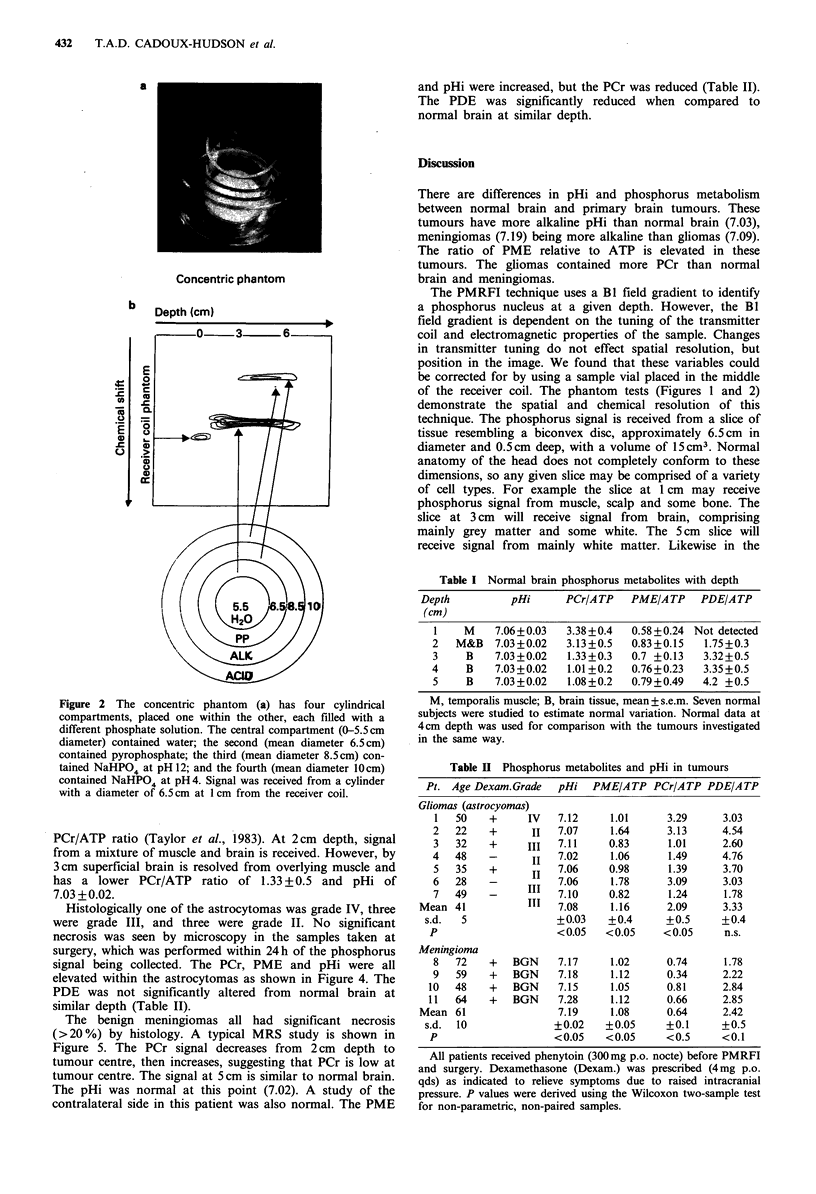
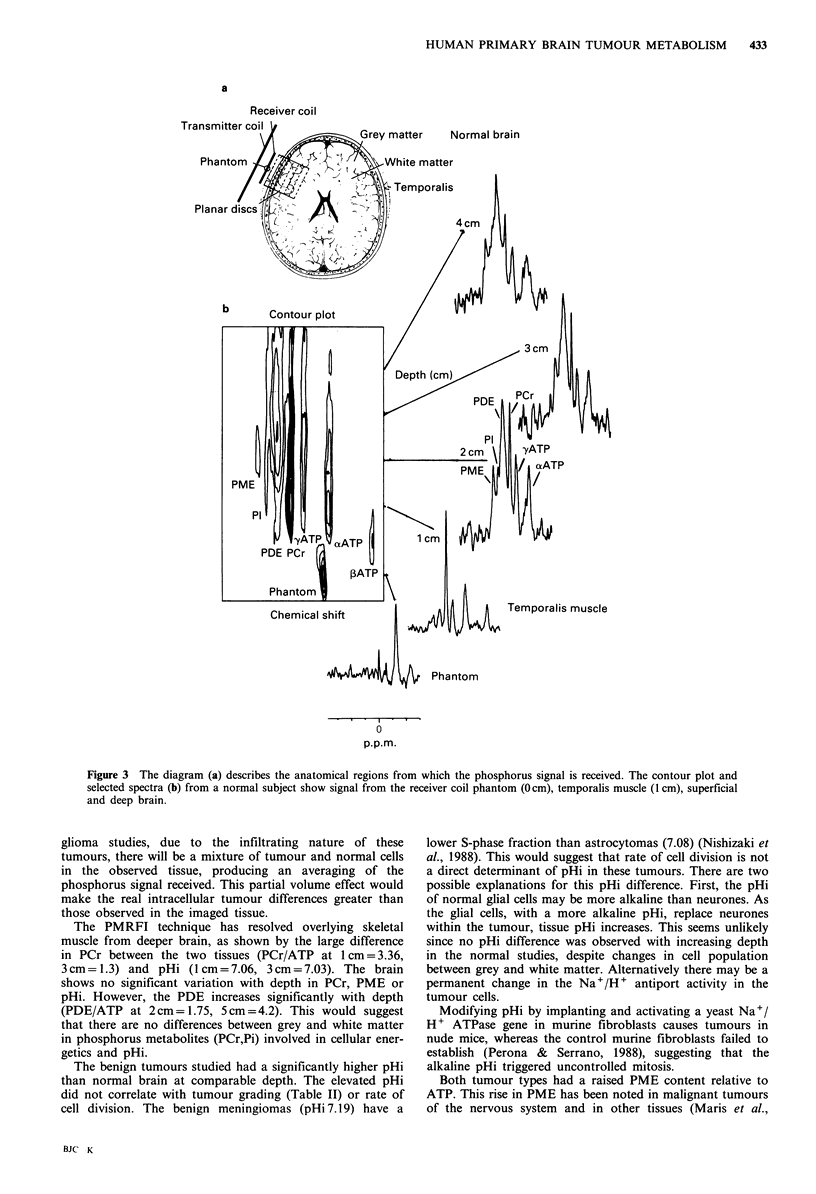
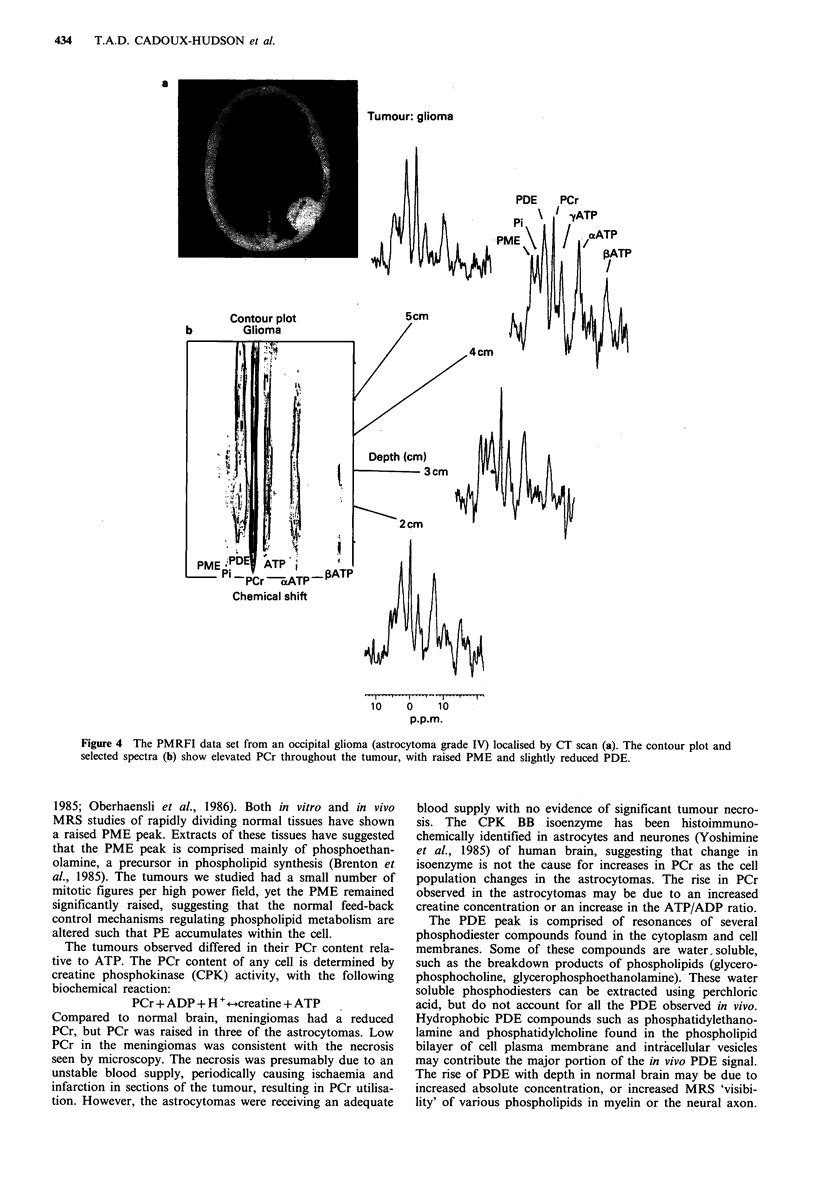
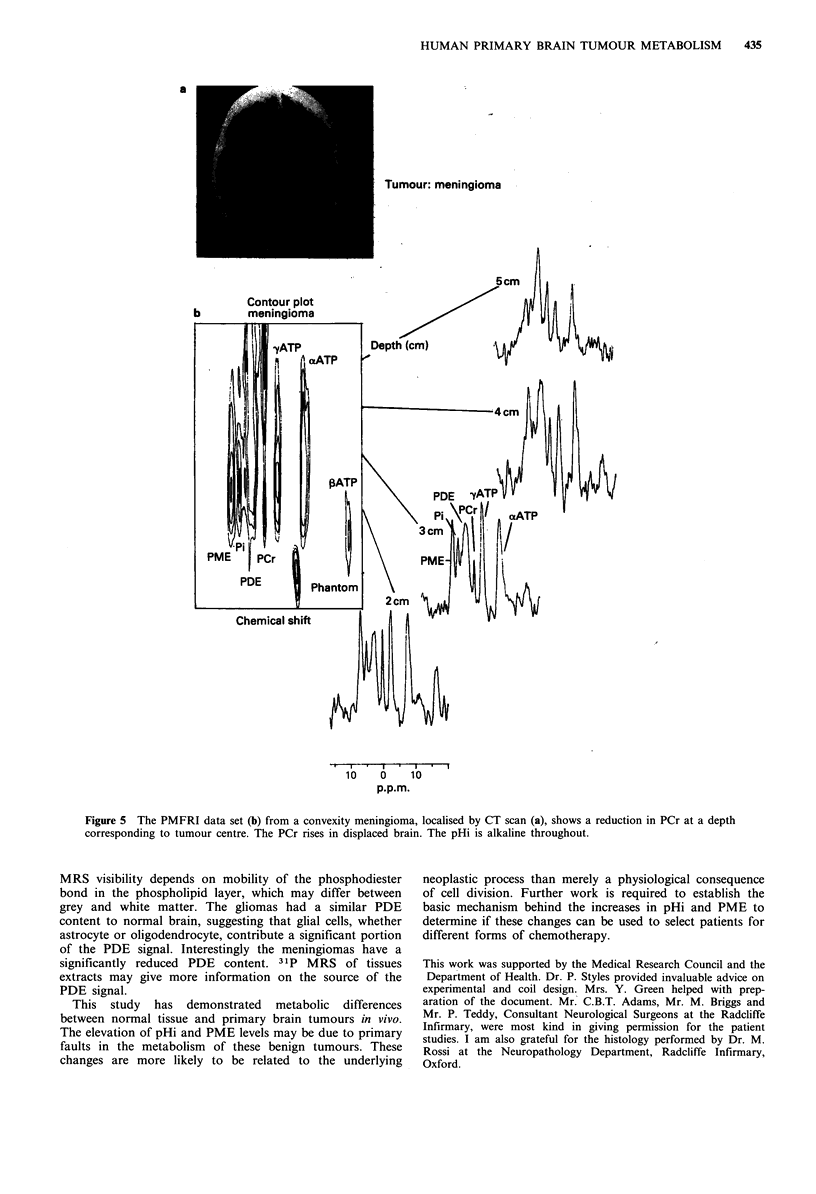
Magnetic resonance spectroscopy was used to study intracellular pH and compounds which contain phosphorus in normal human brain and primary brain tumours non-invasively. In normal subjects (n = 7) intracellular pH (pHi) of the brain was 7.03 +/- 0.02 (mean +/- s.e.m.). The pHi did not vary between superficial (2 cm, majority grey matter) and deep brain (5 cm, majority white matter). The relative concentrations of phosphocreatine (PCr) and phosphomonoesters (PME) to ATP were also constant with depth. The relative concentration of phosphodiesters (PDE) increased from superficial to deep in normal brain. The astrocytomas (n = 7, grade II-IV) were significantly more alkaline (pHi = 7.08 +/- 0.03), and contained more PCr and PME, with respect to ATP, than normal brain at similar depth. The meningiomas (n = 4) were also more alkaline (pHi = 7.19 +/- 0.02) with a raised PME level but reduced PCr. The reduction in meningioma PCr may be due to the significant necrosis (greater than 20%) seen in the surgical biopsies. No significant necrosis was seen in the gliomas. Previous in vitro studies suggest that increased PME may be due to accumulation of phosphoethanolamine (PE), a phospholipid precursor. These results suggest that human primary brain tumours characteristically are more alkaline with increased PME than normal brain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackledge M. J., Rajagopalan B., Oberhaensli R. D., Bolas N. M., Styles P., Radda G. K. Quantitative studies of human cardiac metabolism by 31P rotating-frame NMR. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4283–4287. doi: 10.1073/pnas.84.12.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenton D. P., Garrod P. J., Krywawych S., Reynolds E. O., Bachelard H. S., Cox D. W., Morris P. G. Phosphoethanolamine is major constituent of phosphomonester peak detected by 31P NMR in newborn brain. Lancet. 1985 Jan 12;1(8420):115–115. doi: 10.1016/s0140-6736(85)92012-4. [DOI] [PubMed] [Google Scholar]
- Daly P. F., Lyon R. C., Faustino P. J., Cohen J. S. Phospholipid metabolism in cancer cells monitored by 31P NMR spectroscopy. J Biol Chem. 1987 Nov 5;262(31):14875–14878. [PubMed] [Google Scholar]
- Maris J. M., Evans A. E., McLaughlin A. C., D'Angio G. J., Bolinger L., Manos H., Chance B. 31P nuclear magnetic resonance spectroscopic investigation of human neuroblastoma in situ. N Engl J Med. 1985 Jun 6;312(23):1500–1505. doi: 10.1056/NEJM198506063122307. [DOI] [PubMed] [Google Scholar]
- Miceli M. V., Kan L. S., Newsome D. A. Phosphorus-31 nuclear magnetic resonance spectroscopy of human retinoblastoma cells: correlation with metabolic indices. Biochim Biophys Acta. 1988 Jul 29;970(3):262–269. doi: 10.1016/0167-4889(88)90125-5. [DOI] [PubMed] [Google Scholar]
- Nishizaki T., Orita T., Saiki M., Furutani Y., Aoki H. Cell kinetics studies of human brain tumors by in vitro labeling using anti-BUdR monoclonal antibody. J Neurosurg. 1988 Sep;69(3):371–374. doi: 10.3171/jns.1988.69.3.0371. [DOI] [PubMed] [Google Scholar]
- Oberhaensli R. D., Hilton-Jones D., Bore P. J., Hands L. J., Rampling R. P., Radda G. K. Biochemical investigation of human tumours in vivo with phosphorus-31 magnetic resonance spectroscopy. Lancet. 1986 Jul 5;2(8497):8–11. doi: 10.1016/s0140-6736(86)92558-4. [DOI] [PubMed] [Google Scholar]
- Perona R., Serrano R. Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nature. 1988 Aug 4;334(6181):438–440. doi: 10.1038/334438a0. [DOI] [PubMed] [Google Scholar]
- Radda G. K., Rajagopalan B., Taylor D. J. Biochemistry in vivo: an appraisal of clinical magnetic resonance spectroscopy. Magn Reson Q. 1989 Apr;5(2):122–151. [PubMed] [Google Scholar]
- Radda G. K. The use of NMR spectroscopy for the understanding of disease. Science. 1986 Aug 8;233(4764):640–645. doi: 10.1126/science.3726553. [DOI] [PubMed] [Google Scholar]
- Styles P. Passive electrical isolation of double coil probes for localized spectroscopy and imaging. NMR Biomed. 1988 Apr;1(2):61–66. doi: 10.1002/nbm.1940010202. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Bore P. J., Styles P., Gadian D. G., Radda G. K. Bioenergetics of intact human muscle. A 31P nuclear magnetic resonance study. Mol Biol Med. 1983 Jul;1(1):77–94. [PubMed] [Google Scholar]
- Yoshimine T., Morimoto K., Homburger H. A., Yanagihara T. Immunohistochemical localization of creatine kinase BB-isoenzyme in human brain: comparison with tubulin and astroprotein. Brain Res. 1983 Apr 11;265(1):101–108. doi: 10.1016/0006-8993(83)91338-0. [DOI] [PubMed] [Google Scholar]