Abstract
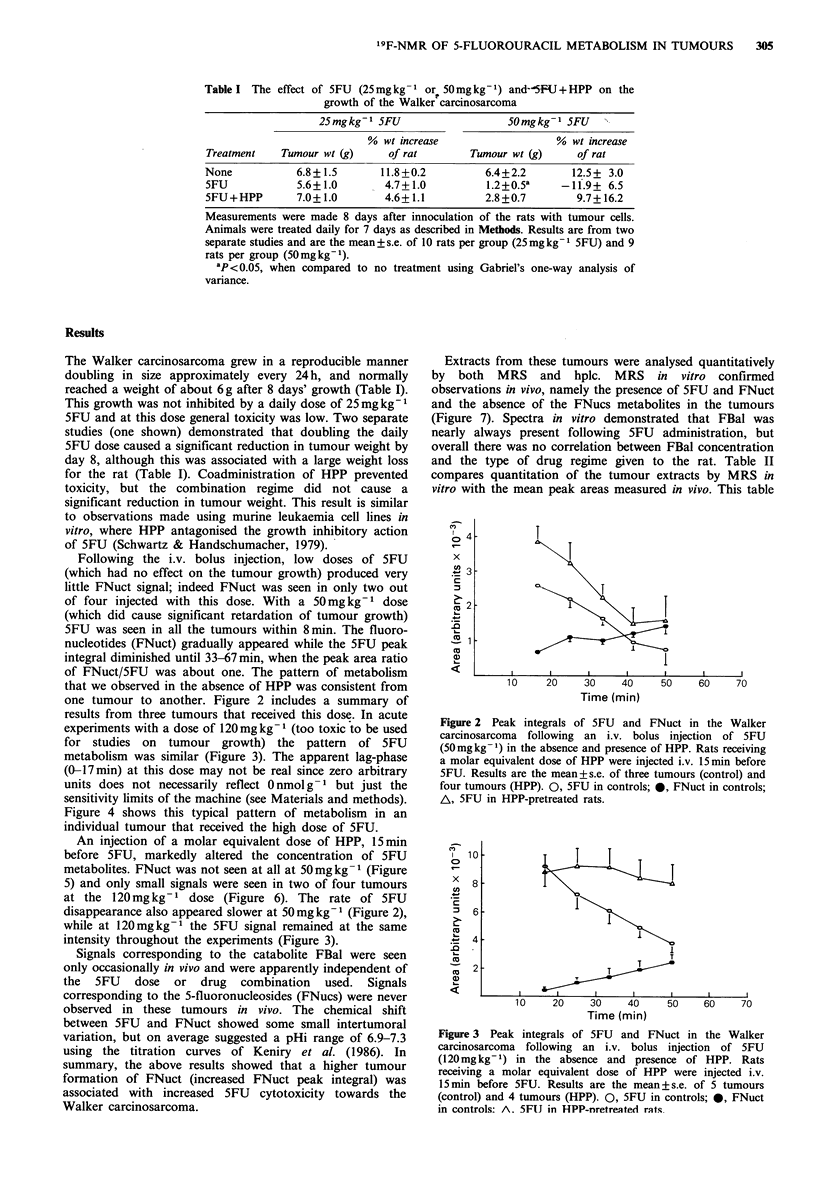
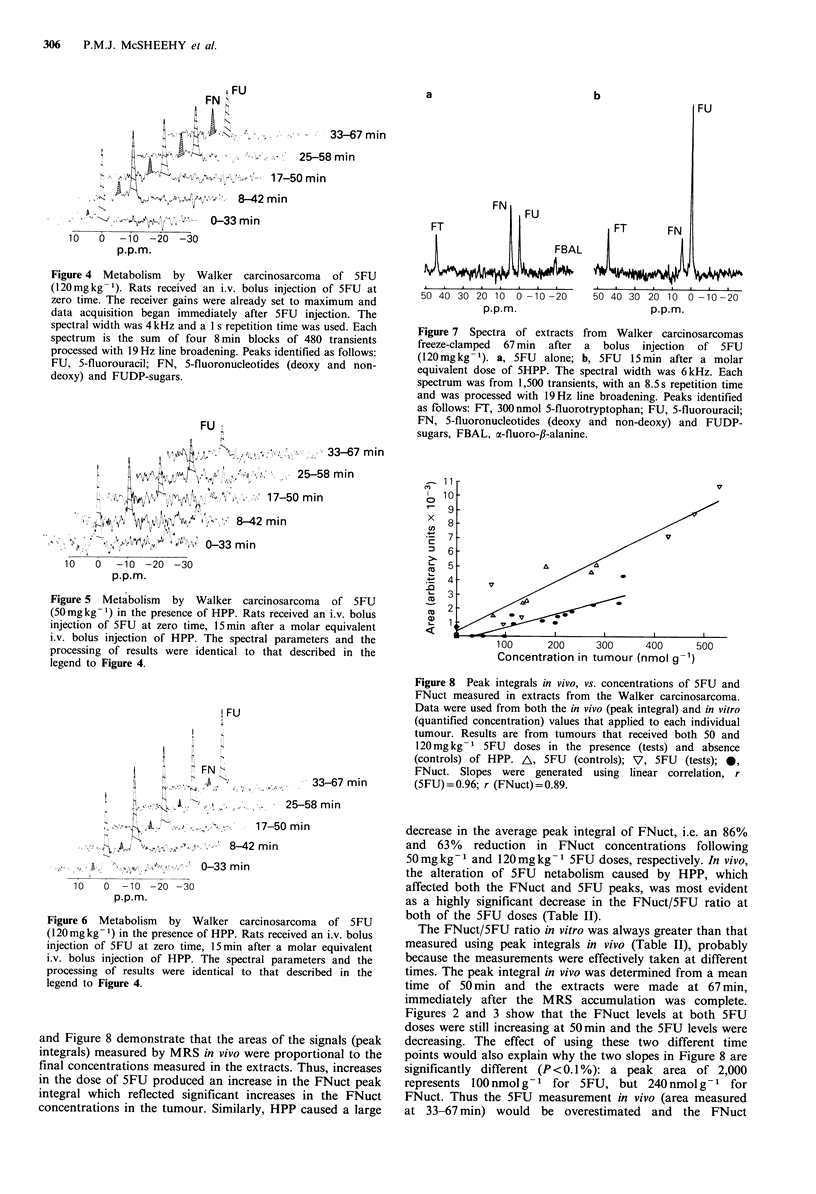
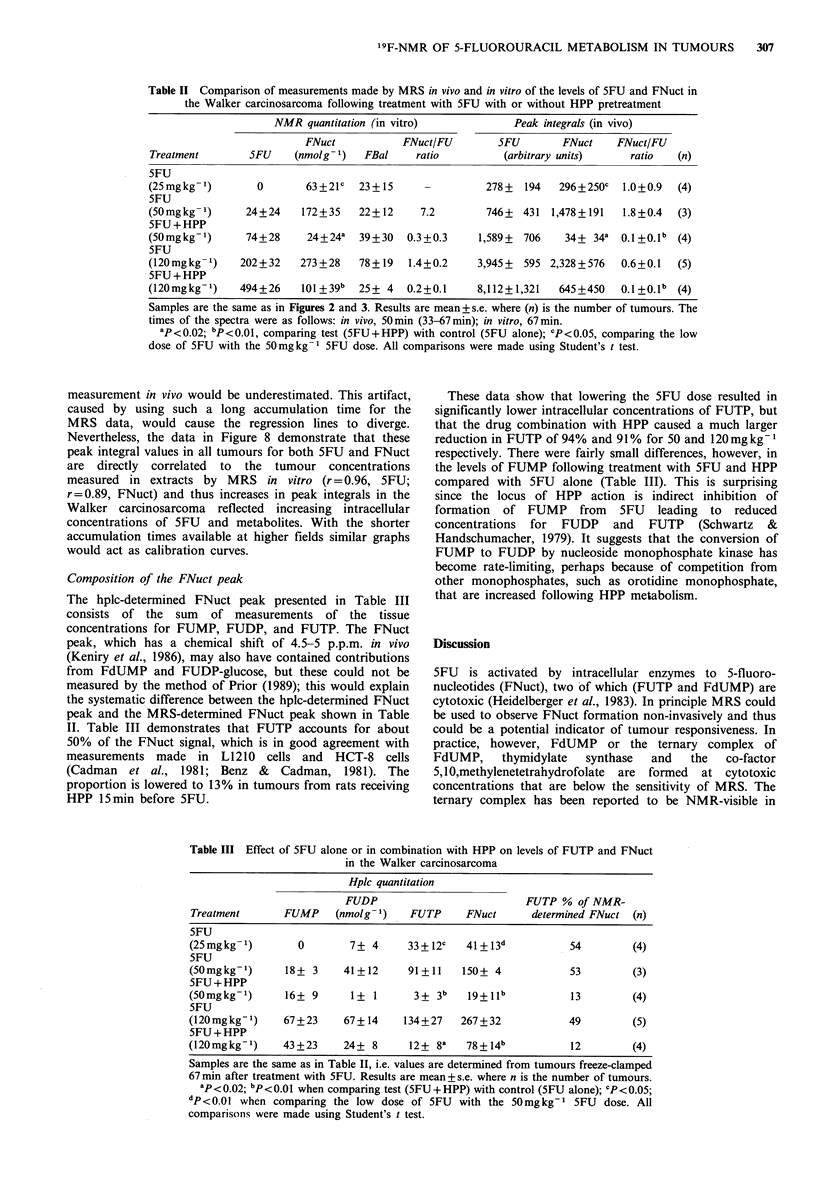
19F-magnetic resonance spectroscopy (MRS) can be used to non-invasively monitor metabolism of 5-fluorouracil (5FU) to cytotoxic fluoronucleotides (FNuct). We investigated whether the levels of FNuct formed from 5FU and observed in vivo by MRS in the Walker carcinosarcoma predicted cytotoxicity. Fifty mg kg-1 5FU caused tumour FNuct formation and, when repeated daily for 1 week, significant tumour growth inhibition (P less than 5%). Twenty-five mg kg-1 5FU produced less tumour FNuct (P less than 5%) and did not cause significant tumour regression. Tumour regression and tumour FNuct formation were also suppressed by 50 mg kg-1 5FU combined with a molar equivalent dose of allopurinol (P less than 2%). Tumour extracts were analysed by hplc and MRS confirming the observations in vivo and demonstrating that peak integrals in vivo were directly proportional to 5FU and FNuct concentrations. Hplc analysis of extracts showed that 50% of FNuct in tumours treated with 5FU was the cytotoxic nucleotide FUTP; this was lowered to 5% by a molar equivalent dose of allopurinol (P less than 2%). Twenty-five mg kg-1 5FU also produced significantly less FUTP (36%) than the 50 mg kg-1 dose (P less than 5%). These results suggest that MRS-detectable changes in tumour FNuct (mostly in FUTP) can be used to predict 5FU cytotoxicity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz C., Cadman E. Modulation of 5-fluorouracil metabolism and cytotoxicity by antimetabolite pretreatment in human colorectal adenocarcinoma HCT-8. Cancer Res. 1981 Mar;41(3):994–999. [PubMed] [Google Scholar]
- Berne M., Gustavsson B., Almersjö O., Spears C. P., Waldenström J. Concurrent allopurinol and 5-fluorouracil: 5-fluoro-2'-deoxyuridylate formation and thymidylate synthase inhibition in rat colon carcinoma and in regenerating rat liver. Cancer Chemother Pharmacol. 1987;20(3):193–197. doi: 10.1007/BF00570483. [DOI] [PubMed] [Google Scholar]
- Cabanac S., Malet-Martino M. C., Bon M., Martino R., Nedelec J. F., Dimicoli J. L. Direct 19F NMR spectroscopic observation of 5-fluorouracil metabolism in the isolated perfused mouse liver model. NMR Biomed. 1988 Jun;1(3):113–120. doi: 10.1002/nbm.1940010303. [DOI] [PubMed] [Google Scholar]
- Cadman E., Heimer R., Benz C. The influence of methotrexate pretreatment on 5-fluorouracil metabolism in L1210 cells. J Biol Chem. 1981 Feb 25;256(4):1695–1704. [PubMed] [Google Scholar]
- Griffiths J. R., Bhujwalla Z., Coombes R. C., Maxwell R. J., Midwood C. J., Morgan R. J., Nias A. H., Perry P., Prior M., Prysor-Jones R. A. Monitoring cancer therapy by NMR spectroscopy. Ann N Y Acad Sci. 1987;508:183–199. doi: 10.1111/j.1749-6632.1987.tb32904.x. [DOI] [PubMed] [Google Scholar]
- Howell S. B., Wung W. E., Taetle R., Hussain F., Romine J. S. Modulation of 5-fluorouracil toxicity by allopurinol in man. Cancer. 1981 Sep 15;48(6):1281–1289. doi: 10.1002/1097-0142(19810915)48:6<1281::aid-cncr2820480603>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Hull W. E., Port R. E., Herrmann R., Britsch B., Kunz W. Metabolites of 5-fluorouracil in plasma and urine, as monitored by 19F nuclear magnetic resonance spectroscopy, for patients receiving chemotherapy with or without methotrexate pretreatment. Cancer Res. 1988 Mar 15;48(6):1680–1688. [PubMed] [Google Scholar]
- Klubes P., Connelly K., Cerna I., Mandel H. G. Effects of 5-fluorouracil on 5-fluorodeoxyuridine 5'-monophosphate and 2-deoxyuridine 5'-monophosphate pools, and DNA synthesis in solid mouse L1210 and rat Walker 256 tumors. Cancer Res. 1978 Aug;38(8):2325–2331. [PubMed] [Google Scholar]
- Mackintosh J., Tattersall M. H. Biochemical modulation of fluorouracil therapy in advanced colorectal cancer. Ann Acad Med Singapore. 1987 Jul;16(3):444–448. [PubMed] [Google Scholar]
- Malet-Martino M. C., Faure F., Vialaneix J. P., Palevody C., Hollande E., Martino R. Noninvasive fluorine-19 NMR study of fluoropyrimidine metabolism in cell cultures of human pancreatic and colon adenocarcinoma. Cancer Chemother Pharmacol. 1986;18(1):5–10. doi: 10.1007/BF00253054. [DOI] [PubMed] [Google Scholar]
- Pinedo H. M., Peters G. F. Fluorouracil: biochemistry and pharmacology. J Clin Oncol. 1988 Oct;6(10):1653–1664. doi: 10.1200/JCO.1988.6.10.1653. [DOI] [PubMed] [Google Scholar]
- Pogolotti A. L., Jr, Nolan P. A., Santi D. V. Methods for the complete analysis of 5-fluorouracil metabolites in cell extracts. Anal Biochem. 1981 Oct;117(1):178–186. doi: 10.1016/0003-2697(81)90708-9. [DOI] [PubMed] [Google Scholar]
- Schwartz P. M., Handschumacher R. E. Selective antagonism of 5-fluorouracil cytotoxicity by 4-hydroxypyrazolopyrimidine (allopurinol) in vitro. Cancer Res. 1979 Aug;39(8):3095–3101. [PubMed] [Google Scholar]
- Spears C. P., Shani J., Shahinian A. H., Wolf W., Heidelberger C., Danenberg P. V. Assay and time course of 5-fluorouracil incorporation into RNA of L1210/0 ascites cells in vivo. Mol Pharmacol. 1985 Feb;27(2):302–307. [PubMed] [Google Scholar]
- Stevens A. N., Morris P. G., Iles R. A., Sheldon P. W., Griffiths J. R. 5-fluorouracil metabolism monitored in vivo by 19F NMR. Br J Cancer. 1984 Jul;50(1):113–117. doi: 10.1038/bjc.1984.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto C. H., Tan Y. Y., Cadman E. C., Armstrong R. D. Correlation between ribosomal RNA production and RNA-directed fluoropyrimidine cytotoxicity. Biochem Pharmacol. 1987 Oct 1;36(19):3243–3248. doi: 10.1016/0006-2952(87)90640-x. [DOI] [PubMed] [Google Scholar]
- Washtien W. L. Comparison of 5-fluorouracil metabolism in two human gastrointestinal tumor cell lines. Cancer Res. 1984 Mar;44(3):909–914. [PubMed] [Google Scholar]
- Wolf W., Albright M. J., Silver M. S., Weber H., Reichardt U., Sauer R. Fluorine-19 NMR spectroscopic studies of the metabolism of 5-fluorouracil in the liver of patients undergoing chemotherapy. Magn Reson Imaging. 1987;5(3):165–169. doi: 10.1016/0730-725x(87)90016-6. [DOI] [PubMed] [Google Scholar]



