Abstract
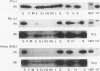
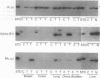
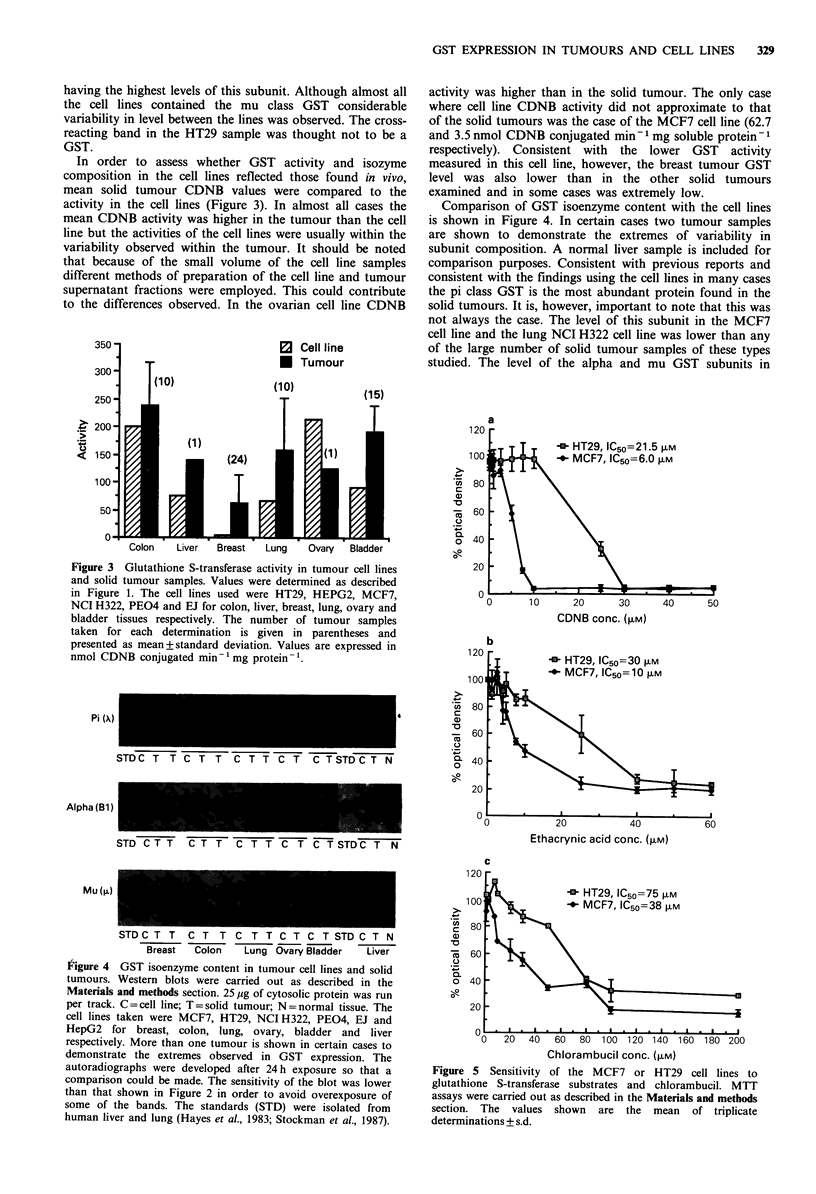
An increasing body of evidence indicates that glutathione S-transferases play a role in the intrinsic and acquired resistance of tumours to anticancer drugs. In view of the wide use of tumour cell lines to understand the factors which confer either sensitivity or resistance to chemotherapeutic agents we have determined glutathione S-transferase (GST) activity and isozyme composition in nine human cell lines. These data have been compared with the values obtained in solid tumours. In most cases overall GST activity was higher in the tumours than in the cell lines. This was most pronounced for the breast tumour samples relative to MCF7 cell line. The pi class GST subunit was present at similar concentration in the cell lines and the tumours, and in most cases was the most abundant subunit present. The alpha and mu class GST were expressed in most of the cell lines but at much lower concentration than the pi class subunit. Also considerable variability particularly in the expression of the mu subunits was observed. This was also the case for the expression of these subunits in the solid tumour samples. The levels of these GSTs (when expressed) in the solid tumours was invariably higher than that observed in the cell lines. There are therefore several similarities but also some significant differences in GST expression in solid tumours and cell lines. Whether the differences are because expression is lost during the generation of the cell lines or whether it reflects the individuality of human tumours remains to be clearly established.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams K. J., Carmichael J., Wolf C. R. Altered mouse bone marrow glutathione and glutathione transferase levels in response to cytotoxins. Cancer Res. 1985 Apr;45(4):1669–1673. [PubMed] [Google Scholar]
- Batist G., Tulpule A., Sinha B. K., Katki A. G., Myers C. E., Cowan K. H. Overexpression of a novel anionic glutathione transferase in multidrug-resistant human breast cancer cells. J Biol Chem. 1986 Nov 25;261(33):15544–15549. [PubMed] [Google Scholar]
- Benson A. M., Barretto P. B. Effects of disulfiram, diethyldithiocarbamate, bisethylxanthogen, and benzyl isothiocyanate on glutathione transferase activities in mouse organs. Cancer Res. 1985 Sep;45(9):4219–4223. [PubMed] [Google Scholar]
- Benson A. M., Batzinger R. P., Ou S. Y., Bueding E., Cha Y. N., Talalay P. Elevation of hepatic glutathione S-transferase activities and protection against mutagenic metabolites of benzo(a)pyrene by dietary antioxidants. Cancer Res. 1978 Dec;38(12):4486–4495. [PubMed] [Google Scholar]
- Buller A. L., Clapper M. L., Tew K. D. Glutathione S-transferases in nitrogen mustard-resistant and -sensitive cell lines. Mol Pharmacol. 1987 Jun;31(6):575–578. [PubMed] [Google Scholar]
- Carmichael J., Adams D. J., Ansell J., Wolf C. R. Glutathione and glutathione transferase levels in mouse granulocytes following cyclophosphamide administration. Cancer Res. 1986 Feb;46(2):735–739. [PubMed] [Google Scholar]
- Carmichael J., DeGraff W. G., Gazdar A. F., Minna J. D., Mitchell J. B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987 Feb 15;47(4):936–942. [PubMed] [Google Scholar]
- Carmichael J., Forrester L. M., Lewis A. D., Hayes J. D., Hayes P. C., Wolf C. R. Glutathione S-transferase isoenzymes and glutathione peroxidase activity in normal and tumour samples from human lung. Carcinogenesis. 1988 Sep;9(9):1617–1621. doi: 10.1093/carcin/9.9.1617. [DOI] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Di Ilio C., Del Boccio G., Aceto A., Casaccia R., Mucilli F., Federici G. Elevation of glutathione transferase activity in human lung tumor. Carcinogenesis. 1988 Feb;9(2):335–340. doi: 10.1093/carcin/9.2.335. [DOI] [PubMed] [Google Scholar]
- Dulik D. M., Fenselau C., Hilton J. Characterization of melphalan-glutathione adducts whose formation is catalyzed by glutathione transferases. Biochem Pharmacol. 1986 Oct 1;35(19):3405–3409. doi: 10.1016/0006-2952(86)90444-2. [DOI] [PubMed] [Google Scholar]
- Evans C. G., Bodell W. J., Tokuda K., Doane-Setzer P., Smith M. T. Glutathione and related enzymes in rat brain tumor cell resistance to 1,3-bis(2-chloroethyl)-1-nitrosourea and nitrogen mustard. Cancer Res. 1987 May 15;47(10):2525–2530. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974 Nov 25;249(22):7130–7139. [PubMed] [Google Scholar]
- Hayes J. D., Gilligan D., Chapman B. J., Beckett G. J. Purification of human hepatic glutathione S-transferases and the development of a radioimmunoassay for their measurement in plasma. Clin Chim Acta. 1983 Oct 31;134(1-2):107–121. doi: 10.1016/0009-8981(83)90189-4. [DOI] [PubMed] [Google Scholar]
- Kitahara A., Satoh K., Nishimura K., Ishikawa T., Ruike K., Sato K., Tsuda H., Ito N. Changes in molecular forms of rat hepatic glutathione S-transferase during chemical hepatocarcinogenesis. Cancer Res. 1984 Jun;44(6):2698–2703. [PubMed] [Google Scholar]
- Kodate C., Fukushi A., Narita T., Kudo H., Soma Y., Sato K. Human placental form of glutathione S-transferase (GST-pi) as a new immunohistochemical marker for human colonic carcinoma. Jpn J Cancer Res. 1986 Mar;77(3):226–229. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis A. D., Hayes J. D., Wolf C. R. Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after the onset of drug resistance: intrinsic differences and cell cycle effects. Carcinogenesis. 1988 Jul;9(7):1283–1287. doi: 10.1093/carcin/9.7.1283. [DOI] [PubMed] [Google Scholar]
- Lewis A. D., Hickson I. D., Robson C. N., Harris A. L., Hayes J. D., Griffiths S. A., Manson M. M., Hall A. E., Moss J. E., Wolf C. R. Amplification and increased expression of alpha class glutathione S-transferase-encoding genes associated with resistance to nitrogen mustards. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8511–8515. doi: 10.1073/pnas.85.22.8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- McGown A. T., Fox B. W. A proposed mechanism of resistance to cyclophosphamide and phosphoramide mustard in a Yoshida cell line in vitro. Cancer Chemother Pharmacol. 1986;17(3):223–226. doi: 10.1007/BF00256688. [DOI] [PubMed] [Google Scholar]
- Pickett C. B., Telakowski-Hopkins C. A., Ding G. J., Argenbright L., Lu A. Y. Rat liver glutathione S-transferases. Complete nucleotide sequence of a glutathione S-transferase mRNA and the regulation of the Ya, Yb, and Yc mRNAs by 3-methylcholanthrene and phenobarbital. J Biol Chem. 1984 Apr 25;259(8):5182–5188. [PubMed] [Google Scholar]
- Robson C. N., Lewis A. D., Wolf C. R., Hayes J. D., Hall A., Proctor S. J., Harris A. L., Hickson I. D. Reduced levels of drug-induced DNA cross-linking in nitrogen mustard-resistant Chinese hamster ovary cells expressing elevated glutathione S-transferase activity. Cancer Res. 1987 Nov 15;47(22):6022–6027. [PubMed] [Google Scholar]
- Shea T. C., Kelley S. L., Henner W. D. Identification of an anionic form of glutathione transferase present in many human tumors and human tumor cell lines. Cancer Res. 1988 Feb 1;48(3):527–533. [PubMed] [Google Scholar]
- Stockman P. K., Beckett G. J., Hayes J. D. Identification of a basic hybrid glutathione S-transferase from human liver. Glutathione S-transferase delta is composed of two distinct subunits (B1 and B2). Biochem J. 1985 Apr 15;227(2):457–465. doi: 10.1042/bj2270457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockman P. K., McLellan L. I., Hayes J. D. Characterization of the basic glutathione S-transferase B1 and B2 subunits from human liver. Biochem J. 1987 May 15;244(1):55–61. doi: 10.1042/bj2440055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew K. D., Bomber A. M., Hoffman S. J. Ethacrynic acid and piriprost as enhancers of cytotoxicity in drug resistant and sensitive cell lines. Cancer Res. 1988 Jul 1;48(13):3622–3625. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Tew K. D. Increased glutathione-S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treat Rep. 1985 Jun;69(6):677–682. [PubMed] [Google Scholar]
- Wolf C. R., Hayward I. P., Lawrie S. S., Buckton K., McIntyre M. A., Adams D. J., Lewis A. D., Scott A. R., Smyth J. F. Cellular heterogeneity and drug resistance in two ovarian adenocarcinoma cell lines derived from a single patient. Int J Cancer. 1987 Jun 15;39(6):695–702. doi: 10.1002/ijc.2910390607. [DOI] [PubMed] [Google Scholar]