Abstract
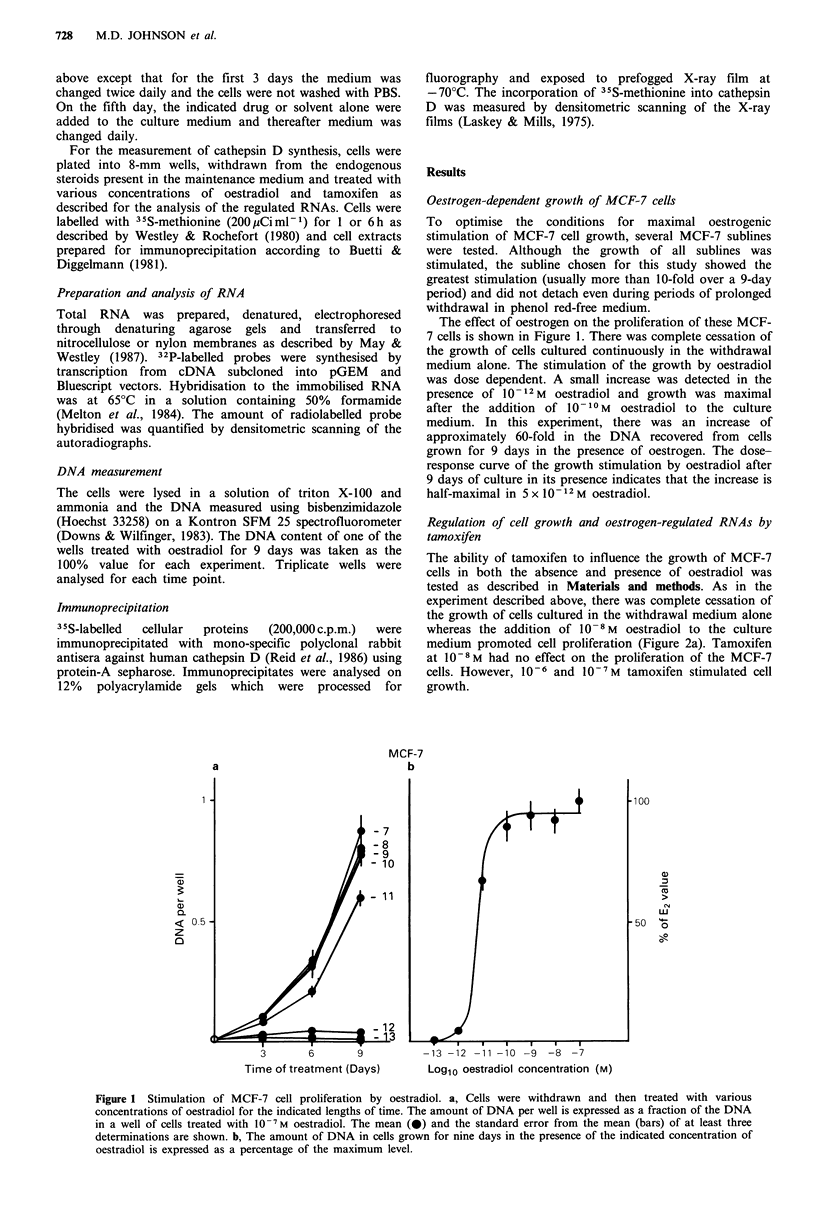
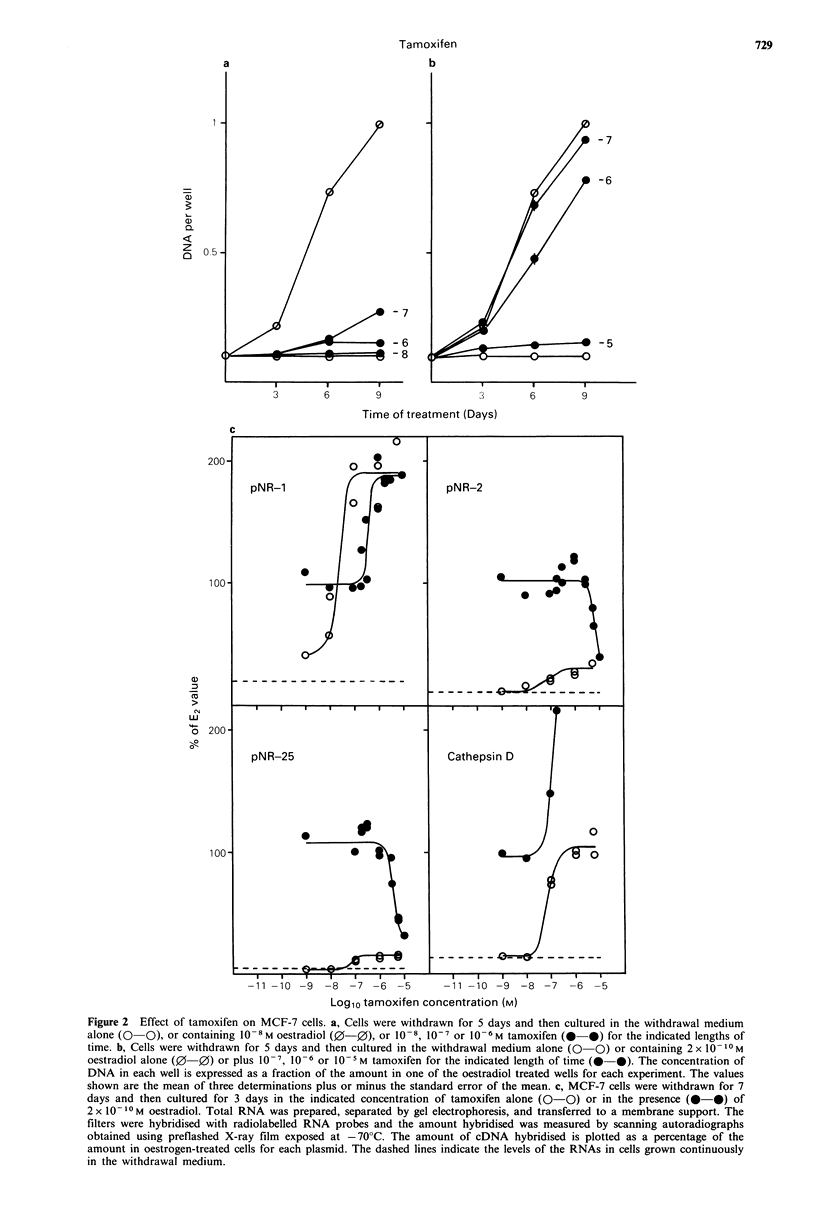
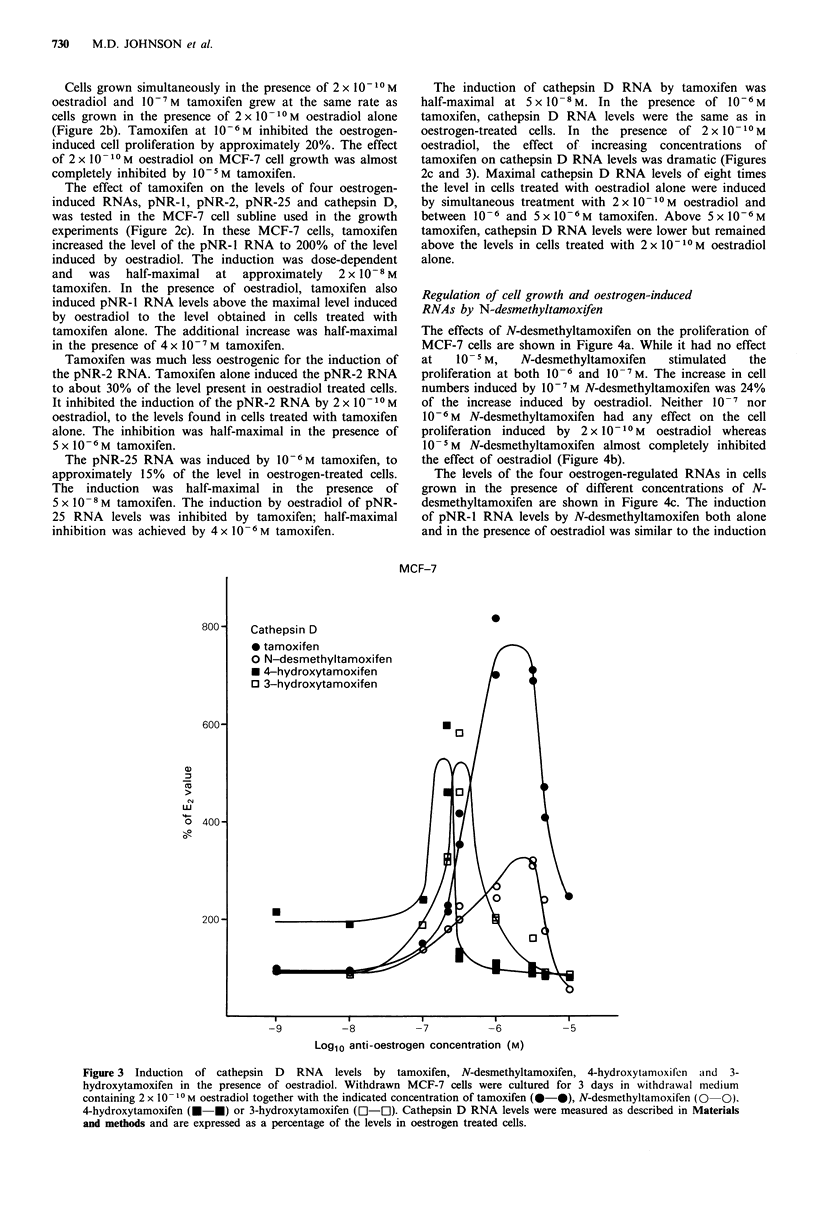
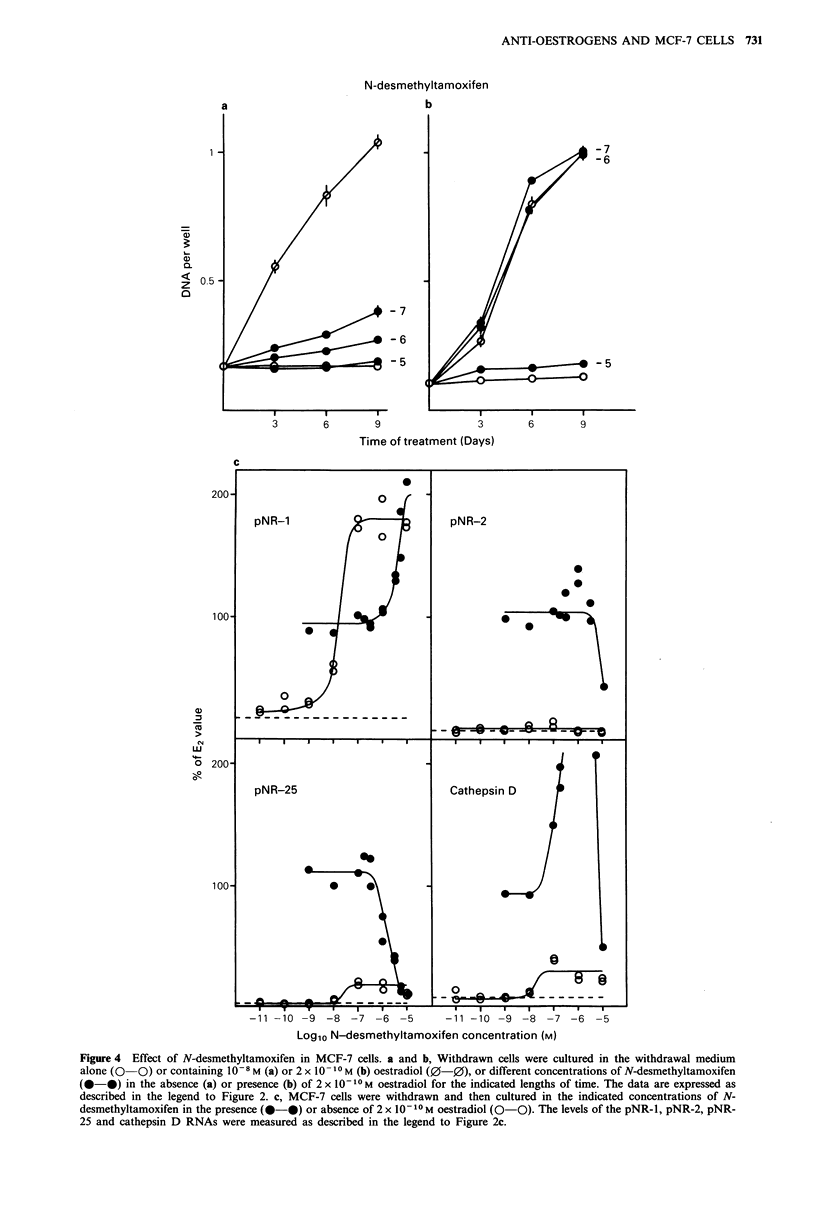
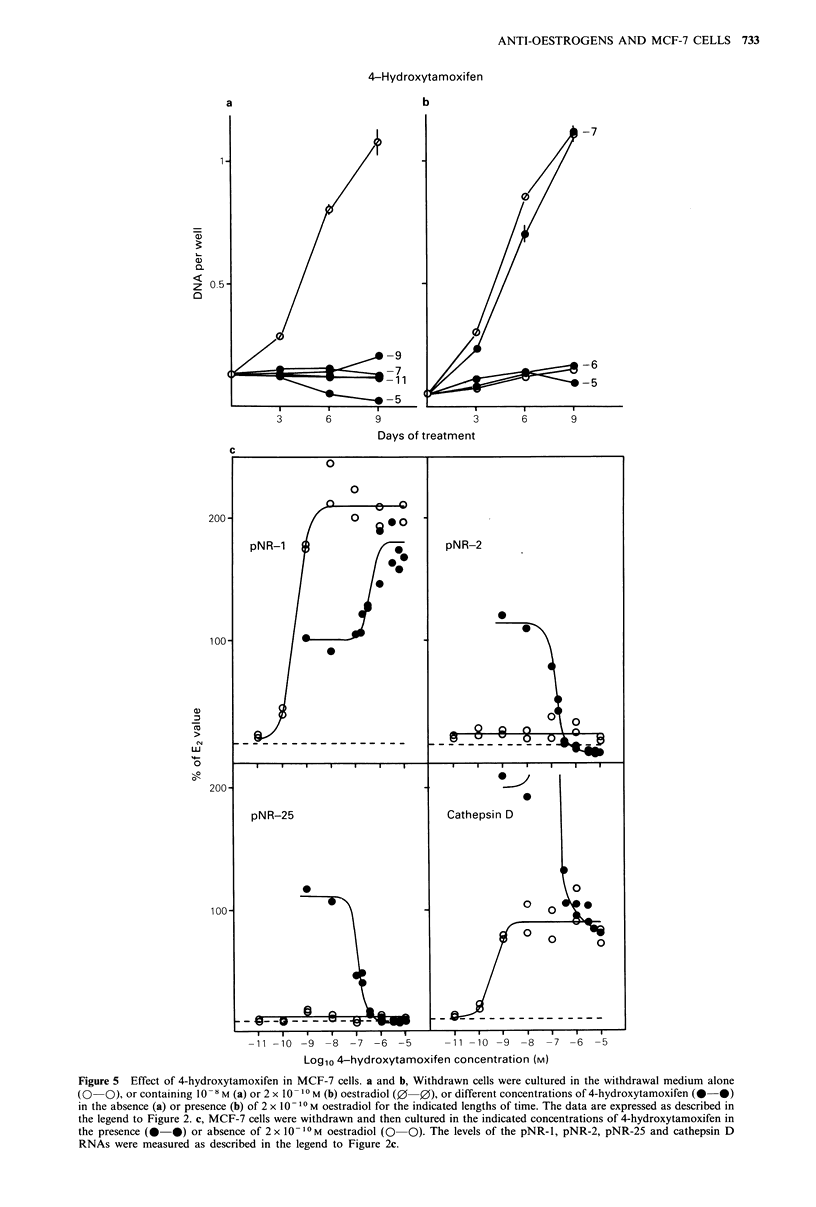
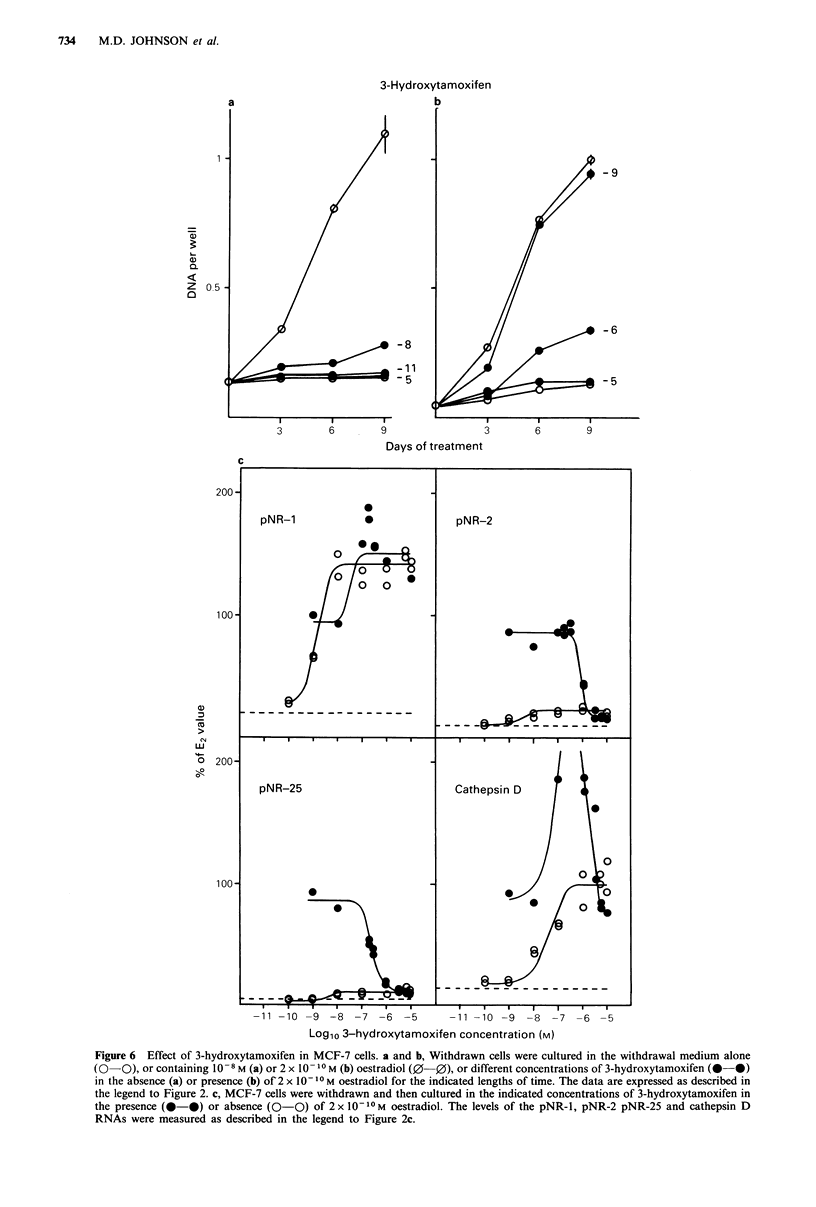
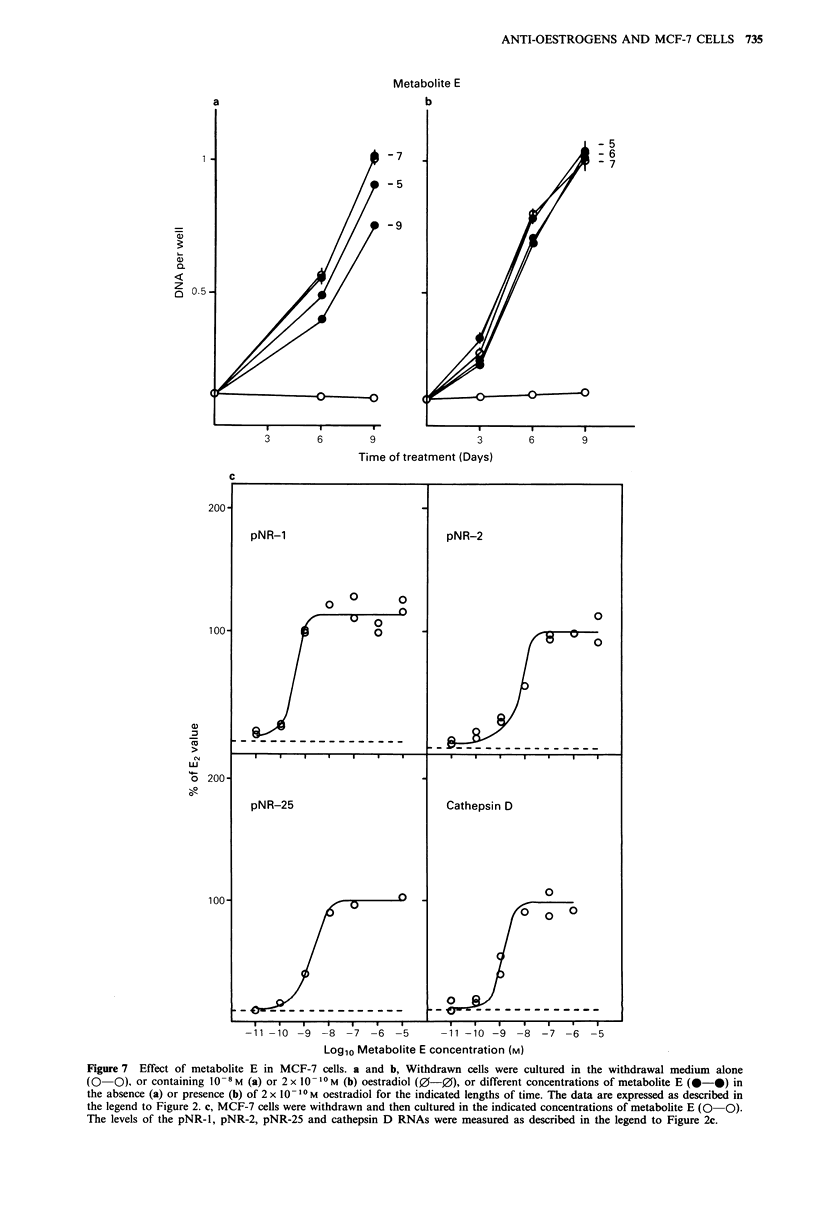
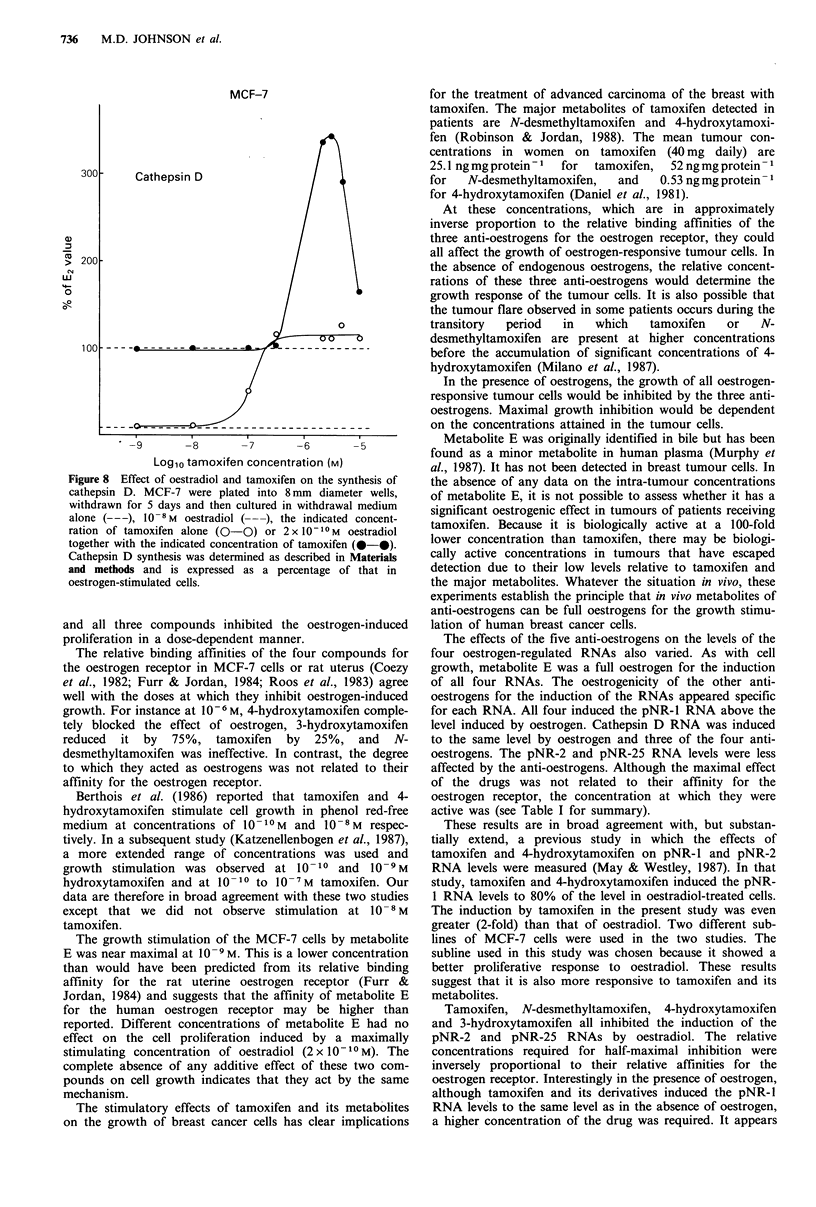
The effects of tamoxifen, three of its in vivo metabolites and 3-hydroxytamoxifen on cellular proliferation and the induction of four oestrogen-regulated RNAs (pNR-1, pNR-2, pNR-25 and cathepsin D) have been measured in MCF-7 breast cancer cells in phenol red-free culture medium. Tamoxifen and 3-hydroxytamoxifen acted as partial oestrogens to stimulate cell growth and the levels of the pNR-2 and pNR-25 RNAs. They were full oestrogens for the induction of cathepsin D RNA and induced the pNR-1 RNA above the level found in oestrogen-treated cells. N-Desmethyltamoxifen and 4-hydroxytamoxifen behaved like tamoxifen except that N-desmethyltamoxifen did not induce the pNR-2 RNA and was only a partial oestrogen for the induction of cathepsin D RNA, and 4-hydroxytamoxifen did not induce the pNR-2 or pNR-25 RNAs. In the presence of oestradiol, the four anti-oestrogens prevented the stimulation of growth and reduced (pNR-2 and pNR-25) or increased (pNR-1) the RNA levels to those present in MCF-7 cells treated with the anti-oestrogen alone. In contrast, for cathepsin D RNA levels there was a synergistic effect of the anti-oestrogens and oestradiol. The concentration at which each anti-oestrogen was effective was related to its affinity for the oestrogen receptor. Metabolite E was a full oestrogen for the induction of cell proliferation and the oestrogen-regulated RNAs. pNR-25 and pNR-2 RNA levels correlated most closely with effects on cell proliferation. These RNAs are therefore potentially the most useful for predicting the response of breast cancer patients to tamoxifen therapy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afting E. G., Becker M. L., Elce J. S. Proteinase and proteinase-inhibitor activities of rat uterine myometrium during pregnancy and involution. Biochem J. 1979 Jan 1;177(1):99–106. doi: 10.1042/bj1770099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briozzo P., Morisset M., Capony F., Rougeot C., Rochefort H. In vitro degradation of extracellular matrix with Mr 52,000 cathepsin D secreted by breast cancer cells. Cancer Res. 1988 Jul 1;48(13):3688–3692. [PubMed] [Google Scholar]
- Buetti E., Diggelmann H. Cloned mouse mammary tumor virus DNA is biologically active in transfected mouse cells and its expression is stimulated by glucocorticoid hormones. Cell. 1981 Feb;23(2):335–345. doi: 10.1016/0092-8674(81)90129-x. [DOI] [PubMed] [Google Scholar]
- Coezy E., Borgna J. L., Rochefort H. Tamoxifen and metabolites in MCF7 cells: correlation between binding to estrogen receptor and inhibition of cell growth. Cancer Res. 1982 Jan;42(1):317–323. [PubMed] [Google Scholar]
- Daniel P., Gaskell S. J., Bishop H., Campbell C., Nicholson R. I. Determination of tamoxifen and biologically active metabolites in human breast tumours and plasma. Eur J Cancer Clin Oncol. 1981 Nov;17(11):1183–1189. [PubMed] [Google Scholar]
- Downs T. R., Wilfinger W. W. Fluorometric quantification of DNA in cells and tissue. Anal Biochem. 1983 Jun;131(2):538–547. doi: 10.1016/0003-2697(83)90212-9. [DOI] [PubMed] [Google Scholar]
- Furr B. J., Jordan V. C. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25(2):127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Zava D. T., Thilagar A. K., Jensen E. M., McGuire W. L. Steroid receptor analyses of nine human breast cancer cell lines. Cancer Res. 1978 Aug;38(8):2434–2437. [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Kendra K. L., Norman M. J., Berthois Y. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Res. 1987 Aug 15;47(16):4355–4360. [PubMed] [Google Scholar]
- Kumar V., Chambon P. The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer. Cell. 1988 Oct 7;55(1):145–156. doi: 10.1016/0092-8674(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May F. E., Westley B. R. Cloning of estrogen-regulated messenger RNA sequences from human breast cancer cells. Cancer Res. 1986 Dec;46(12 Pt 1):6034–6040. [PubMed] [Google Scholar]
- May F. E., Westley B. R. Effects of tamoxifen and 4-hydroxytamoxifen on the pNR-1 and pNR-2 estrogen-regulated RNAs in human breast cancer cells. J Biol Chem. 1987 Nov 25;262(33):15894–15899. [PubMed] [Google Scholar]
- May F. E., Westley B. R. Identification and characterization of estrogen-regulated RNAs in human breast cancer cells. J Biol Chem. 1988 Sep 15;263(26):12901–12908. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano G., Etienne M. C., Frenay M., Khater R., Formento J. L., Renee N., Moll J. L., Francoual M., Berto M., Namer M. Optimised analysis of tamoxifen and its main metabolites in the plasma and cytosol of mammary tumours. Br J Cancer. 1987 May;55(5):509–512. doi: 10.1038/bjc.1987.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy C., Fotsis T., Pantzar P., Adlercreutz H., Martin F. Analysis of tamoxifen and its metabolites in human plasma by gas chromatography-mass spectrometry (GC-MS) using selected ion monitoring (SIM). J Steroid Biochem. 1987 May;26(5):547–555. doi: 10.1016/0022-4731(87)90006-9. [DOI] [PubMed] [Google Scholar]
- Reid W. A., Valler M. J., Kay J. Immunolocalization of cathepsin D in normal and neoplastic human tissues. J Clin Pathol. 1986 Dec;39(12):1323–1330. doi: 10.1136/jcp.39.12.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Daniel J. Y., Tomasetto C., Lathe R., Chenard M. P., Batzenschlager A., Chambon P. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988 Aug 5;241(4866):705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Gairard B., Rasmussen U. B., Krust A., Koehl C., Calderoli H., Schiff V., Renaud R., Chambon P. Specific expression of the pS2 gene in subclasses of breast cancers in comparison with expression of the estrogen and progesterone receptors and the oncogene ERBB2. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9243–9247. doi: 10.1073/pnas.84.24.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Jordan V. C. Metabolism of steroid-modifying anticancer agents. Pharmacol Ther. 1988;36(1):41–103. doi: 10.1016/0163-7258(88)90112-x. [DOI] [PubMed] [Google Scholar]
- Roos W., Oeze L., Löser R., Eppenberger U. Antiestrogenic action of 3-hydroxytamoxifen in the human breast cancer cell line MCF-7. J Natl Cancer Inst. 1983 Jul;71(1):55–59. [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Westley B. R., May F. E. Oestrogen regulates cathepsin D mRNA levels in oestrogen responsive human breast cancer cells. Nucleic Acids Res. 1987 May 11;15(9):3773–3786. doi: 10.1093/nar/15.9.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westley B., Rochefort H. A secreted glycoprotein induced by estrogen in human breast cancer cell lines. Cell. 1980 Jun;20(2):353–362. doi: 10.1016/0092-8674(80)90621-2. [DOI] [PubMed] [Google Scholar]


