Abstract
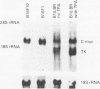
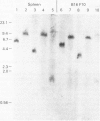
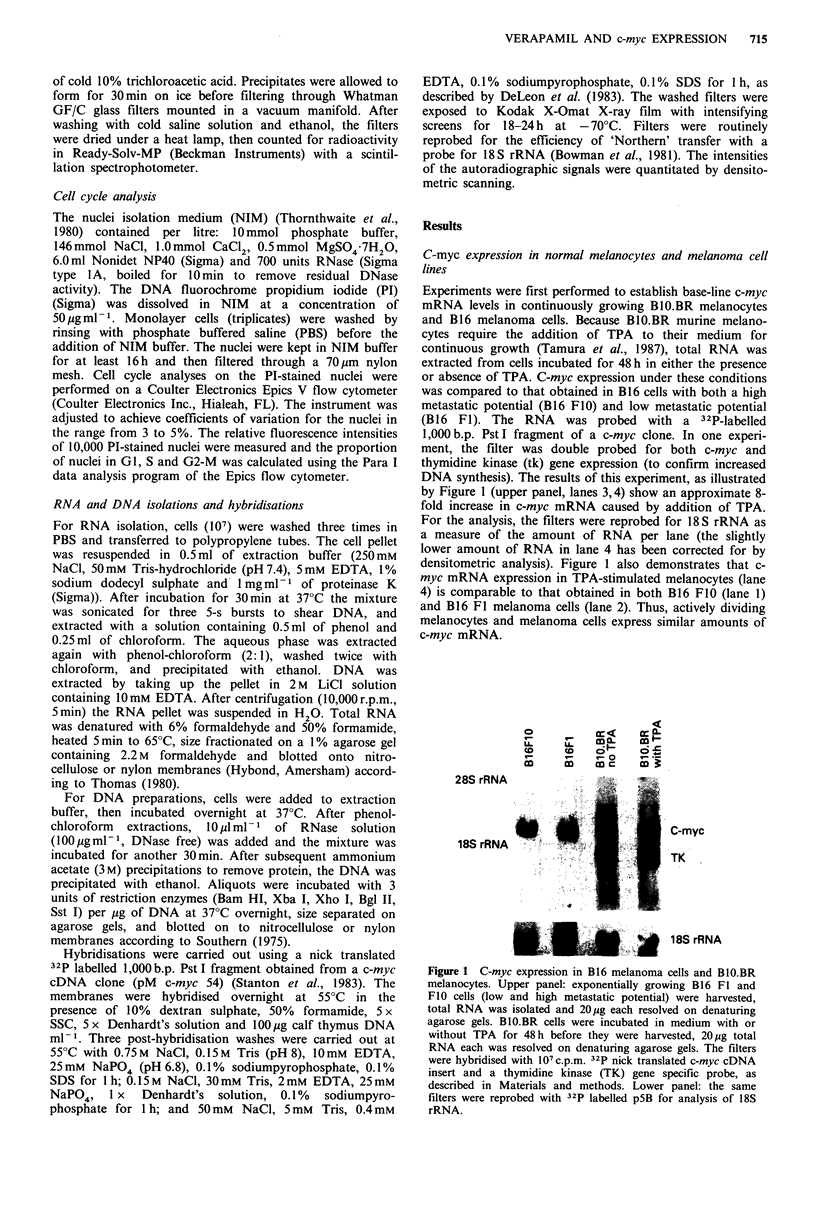
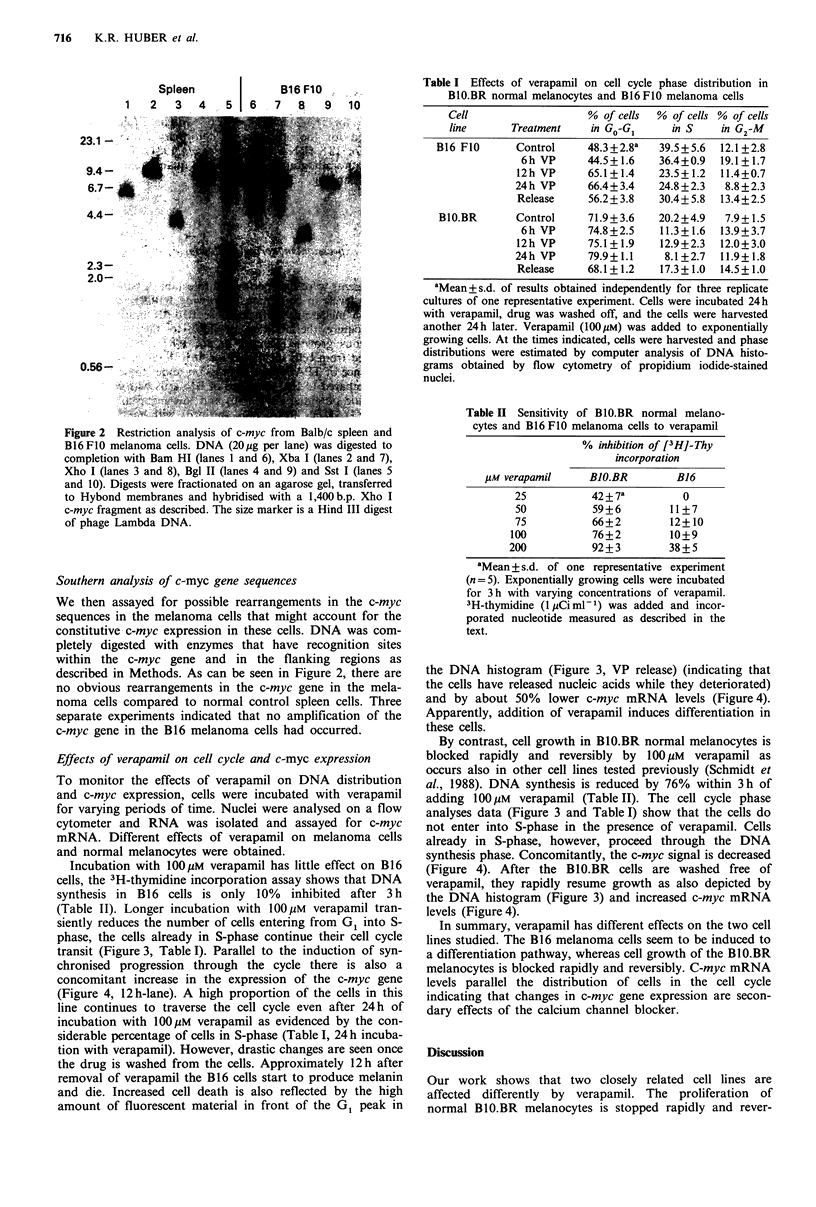
Verapamil, the prototype calcium channel blocker, reversibly inhibits cell proliferation in many normal and tumour cell lines (Schmidt et al., Cancer Res., 48, 3617, 1988). We have found that two closely related cell lines - B16 murine melanoma cells and B10.BR normal murine melanocytes growing in culture - behave differently in the presence of verapamil, and we are now utilising these two related cell lines to help elucidate the molecular basis of verapamil's antiproliferative effect. In this study, we studied cell cycle phase distribution and c-myc gene expression in both cell lines in the absence of verapamil, during incubation with verapamil and after the cells were washed free of verapamil. Our studies show that 100 microM verapamil rapidly blocks DNA synthesis in melanocytes but not in B16 cells. Similarly, incubation with verapamil for 6-24 h results in a decreased c-myc signal in melanocytes, but a transient increase in c-myc expression in B16 cells. After verapamil is washed from the cells following a 24-h incubation with drug, c-myc expression increases in melanocytes as they begin again to proliferate, but decreases in B16 cells as they begin to die. Our disparate results with these cell lines suggest that c-myc gene expression, regardless of its known involvement in growth control, is not the immediate target for verapamil's inhibitory action.
Full text
PDF
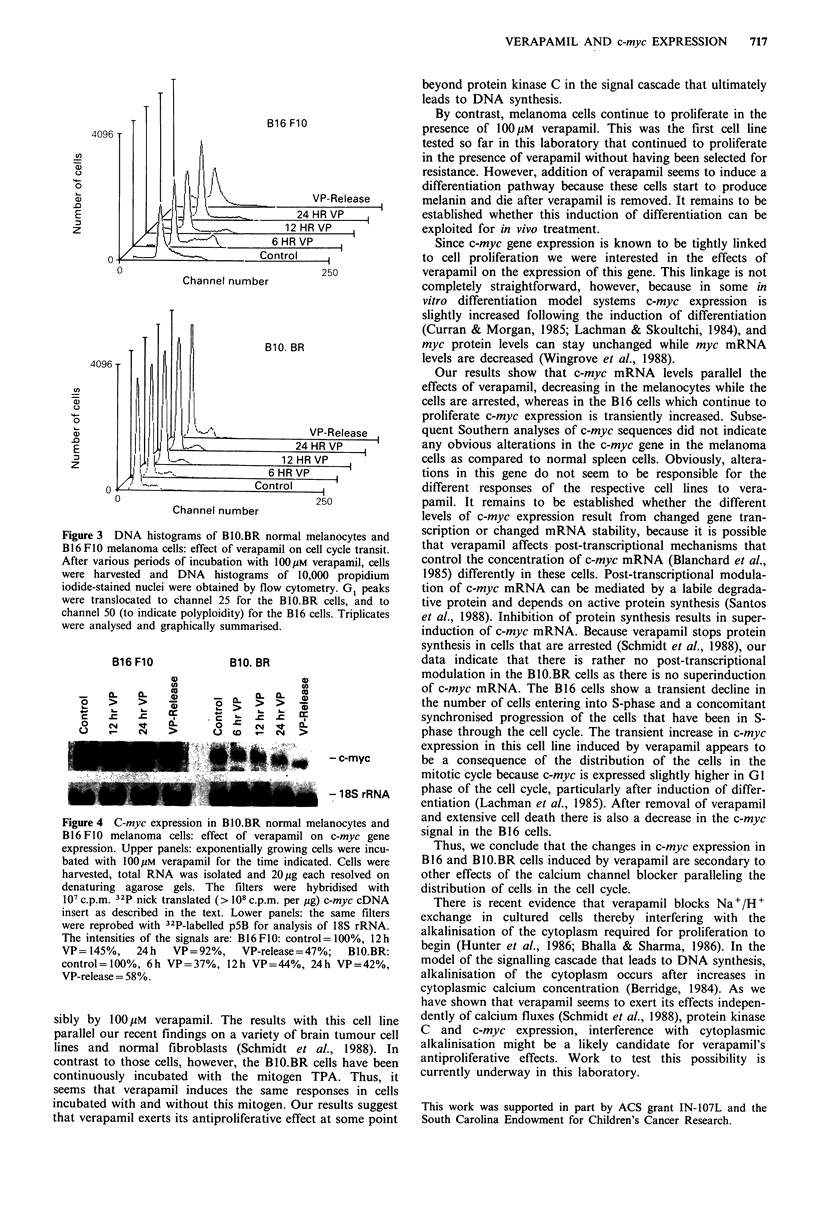

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhalla R. C., Sharma R. V. Competitive interaction of amiloride and verapamil with alpha 1-adrenoceptors in vascular smooth muscle. J Cardiovasc Pharmacol. 1986 Sep-Oct;8(5):927–932. doi: 10.1097/00005344-198609000-00007. [DOI] [PubMed] [Google Scholar]
- Blanchard J. M., Piechaczyk M., Dani C., Chambard J. C., Franchi A., Pouyssegur J., Jeanteur P. c-myc gene is transcribed at high rate in G0-arrested fibroblasts and is post-transcriptionally regulated in response to growth factors. Nature. 1985 Oct 3;317(6036):443–445. doi: 10.1038/317443a0. [DOI] [PubMed] [Google Scholar]
- Bowman L. H., Rabin B., Schlessinger D. Multiple ribosomal RNA cleavage pathways in mammalian cells. Nucleic Acids Res. 1981 Oct 10;9(19):4951–4966. doi: 10.1093/nar/9.19.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. D. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- Curran T., Morgan J. I. Superinduction of c-fos by nerve growth factor in the presence of peripherally active benzodiazepines. Science. 1985 Sep 20;229(4719):1265–1268. doi: 10.1126/science.4035354. [DOI] [PubMed] [Google Scholar]
- DeLeon D. V., Cox K. H., Angerer L. M., Angerer R. C. Most early-variant histone mRNA is contained in the pronucleus of sea urchin eggs. Dev Biol. 1983 Nov;100(1):197–206. doi: 10.1016/0012-1606(83)90211-7. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Kripke M. L. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977 Aug 26;197(4306):893–895. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Goknur A. B., Hegge J. O., Berkoff H. A. Control of thallium and sodium fluxes in isolated adult rat heart cells by anthopleurin-A, verapamil and magnesium. J Mol Cell Cardiol. 1986 Nov;18(11):1125–1132. doi: 10.1016/s0022-2828(86)80038-4. [DOI] [PubMed] [Google Scholar]
- Ince P., Appleton D. R., Finney K. J., Sunter J. P., Watson A. J. Verapamil increases the sensitivity of primary human colorectal carcinoma tissue to vincristine. Br J Cancer. 1986 Jan;53(1):137–139. doi: 10.1038/bjc.1986.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Lachman H. M., Hatton K. S., Skoultchi A. I., Schildkraut C. L. c-myc mRNA levels in the cell cycle change in mouse erythroleukemia cells following inducer treatment. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5323–5327. doi: 10.1073/pnas.82.16.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman H. M., Skoultchi A. I. Expression of c-myc changes during differentiation of mouse erythroleukaemia cells. Nature. 1984 Aug 16;310(5978):592–594. doi: 10.1038/310592a0. [DOI] [PubMed] [Google Scholar]
- Merry S., Fetherston C. A., Kaye S. B., Freshney R. I., Plumb J. A. Resistance of human glioma to adriamycin in vitro: the role of membrane transport and its circumvention with verapamil. Br J Cancer. 1986 Jan;53(1):129–135. doi: 10.1038/bjc.1986.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. A., Clutterbuck R. D., Millar J. L., McElwain T. J. Verapamil potentiation of melphalan cytotoxicity and cellular uptake in murine fibrosarcoma and bone marrow. Br J Cancer. 1985 Dec;52(6):813–822. doi: 10.1038/bjc.1985.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Mendoza S. A. Synergistic signals in mitogenesis: role of ion fluxes, cyclic nucleotides and protein kinase C in Swiss 3T3 cells. J Cell Sci Suppl. 1985;3:229–242. doi: 10.1242/jcs.1985.supplement_3.20. [DOI] [PubMed] [Google Scholar]
- Santos G. F., Scott G. K., Lee W. M., Liu E., Benz C. Estrogen-induced post-transcriptional modulation of c-myc proto-oncogene expression in human breast cancer cells. J Biol Chem. 1988 Jul 15;263(20):9565–9568. [PubMed] [Google Scholar]
- Schmidt W. F., Huber K. R., Ettinger R. S., Neuberg R. W. Antiproliferative effect of verapamil alone on brain tumor cells in vitro. Cancer Res. 1988 Jul 1;48(13):3617–3621. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanton L. W., Watt R., Marcu K. B. Translocation, breakage and truncated transcripts of c-myc oncogene in murine plasmacytomas. Nature. 1983 Jun 2;303(5916):401–406. doi: 10.1038/303401a0. [DOI] [PubMed] [Google Scholar]
- Studzinski G. P., Brelvi Z. S., Feldman S. C., Watt R. A. Participation of c-myc protein in DNA synthesis of human cells. Science. 1986 Oct 24;234(4775):467–470. doi: 10.1126/science.3532322. [DOI] [PubMed] [Google Scholar]
- Tamura A., Halaban R., Moellmann G., Cowan J. M., Lerner M. R., Lerner A. B. Normal murine melanocytes in culture. In Vitro Cell Dev Biol. 1987 Jul;23(7):519–522. doi: 10.1007/BF02628423. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornthwaite J. T., Sugarbaker E. V., Temple W. J. Preparation of tissues for DNA flow cytometric analysis. Cytometry. 1980 Nov;1(3):229–237. doi: 10.1002/cyto.990010309. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Nojiri M., Tsukagoshi S., Sakurai Y. Circumvention of vincristine and Adriamycin resistance in vitro and in vivo by calcium influx blockers. Cancer Res. 1983 Jun;43(6):2905–2910. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Increased accumulation of vincristine and adriamycin in drug-resistant P388 tumor cells following incubation with calcium antagonists and calmodulin inhibitors. Cancer Res. 1982 Nov;42(11):4730–4733. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Overcoming of vincristine resistance in P388 leukemia in vivo and in vitro through enhanced cytotoxicity of vincristine and vinblastine by verapamil. Cancer Res. 1981 May;41(5):1967–1972. [PubMed] [Google Scholar]
- Tsuruo T., Iida H., Tsukagoshi S., Sakurai Y. Potentiation of vincristine and Adriamycin effects in human hemopoietic tumor cell lines by calcium antagonists and calmodulin inhibitors. Cancer Res. 1983 May;43(5):2267–2272. [PubMed] [Google Scholar]
- Wingrove T. G., Watt R., Keng P., Macara I. G. Stabilization of myc proto-oncogene proteins during Friend murine erythroleukemia cell differentiation. J Biol Chem. 1988 Jun 25;263(18):8918–8924. [PubMed] [Google Scholar]
- Yalowich J. C., Ross W. E. Potentiation of etoposide-induced DNA damage by calcium antagonists in L1210 cells in vitro. Cancer Res. 1984 Aug;44(8):3360–3365. [PubMed] [Google Scholar]
- Yalowich J. C., Ross W. E. Verapamil-induced augmentation of etoposide accumulation in L1210 cells in vitro. Cancer Res. 1985 Apr;45(4):1651–1656. [PubMed] [Google Scholar]