Abstract
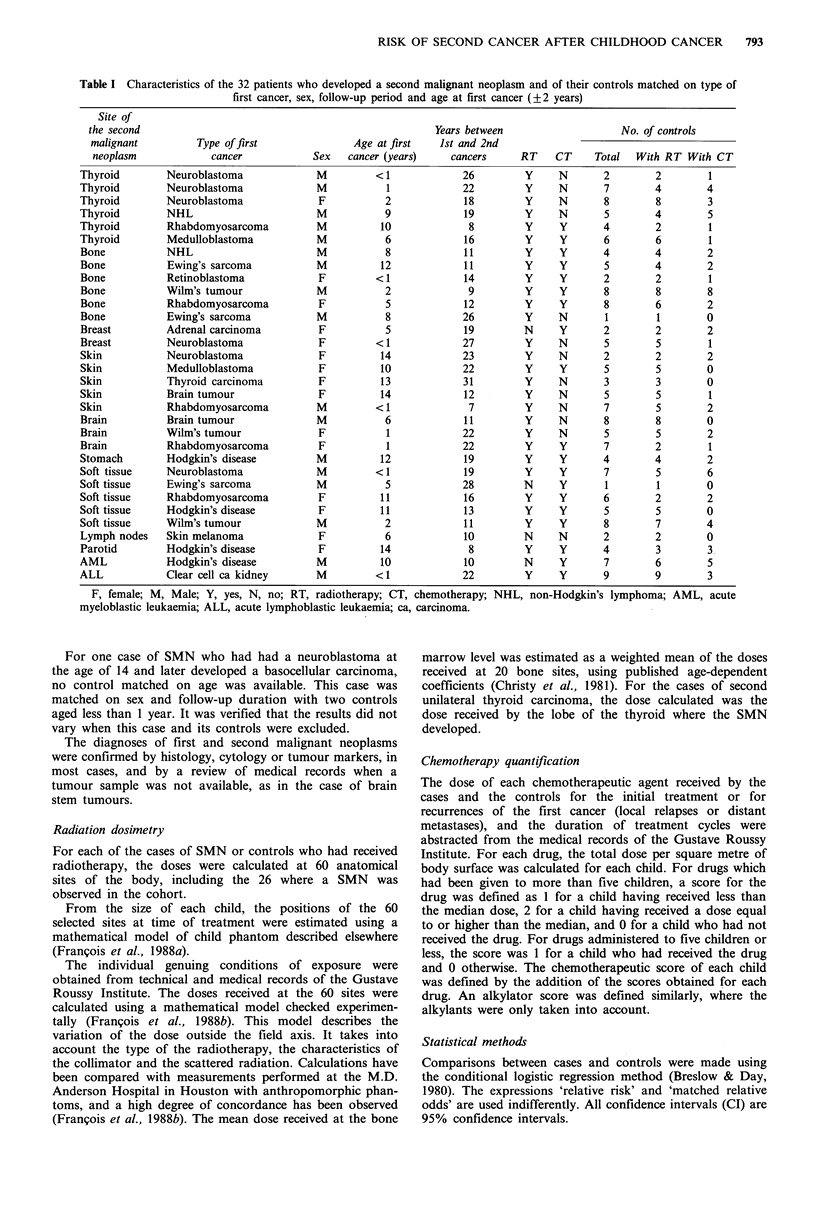
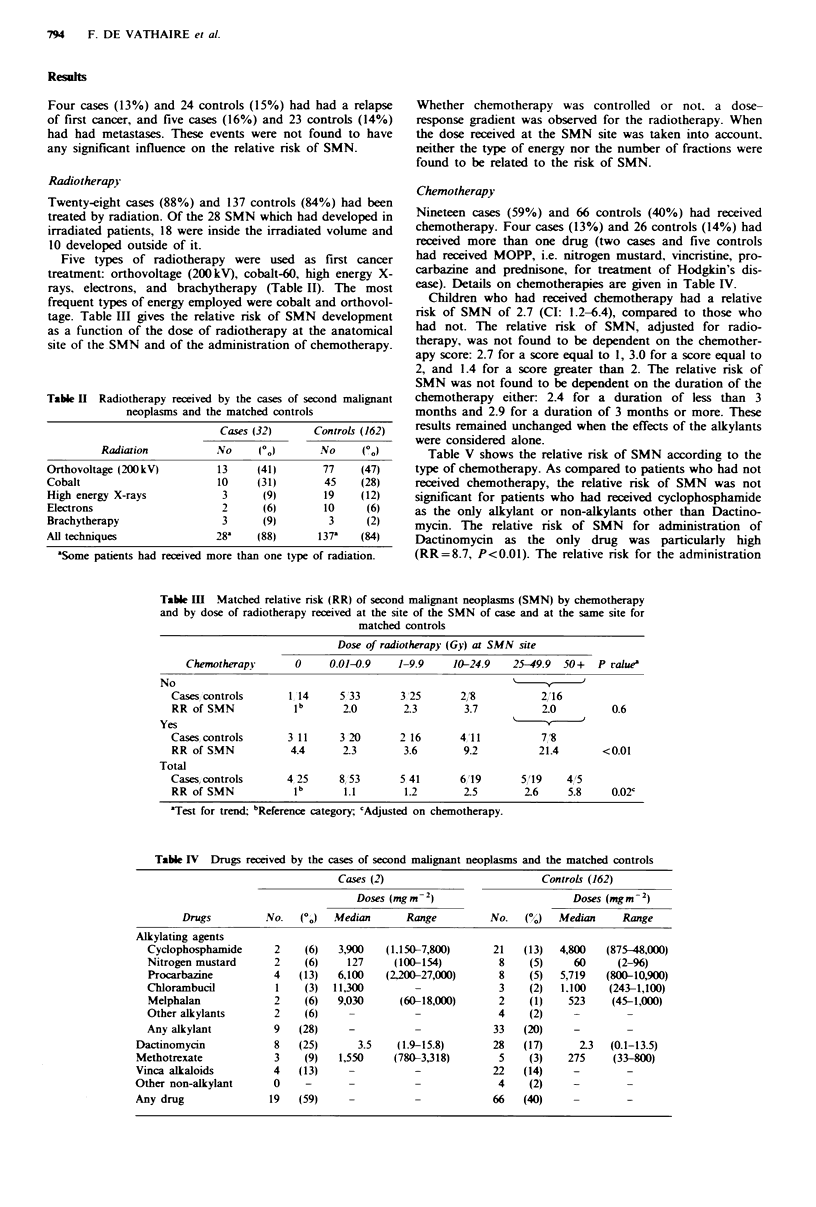
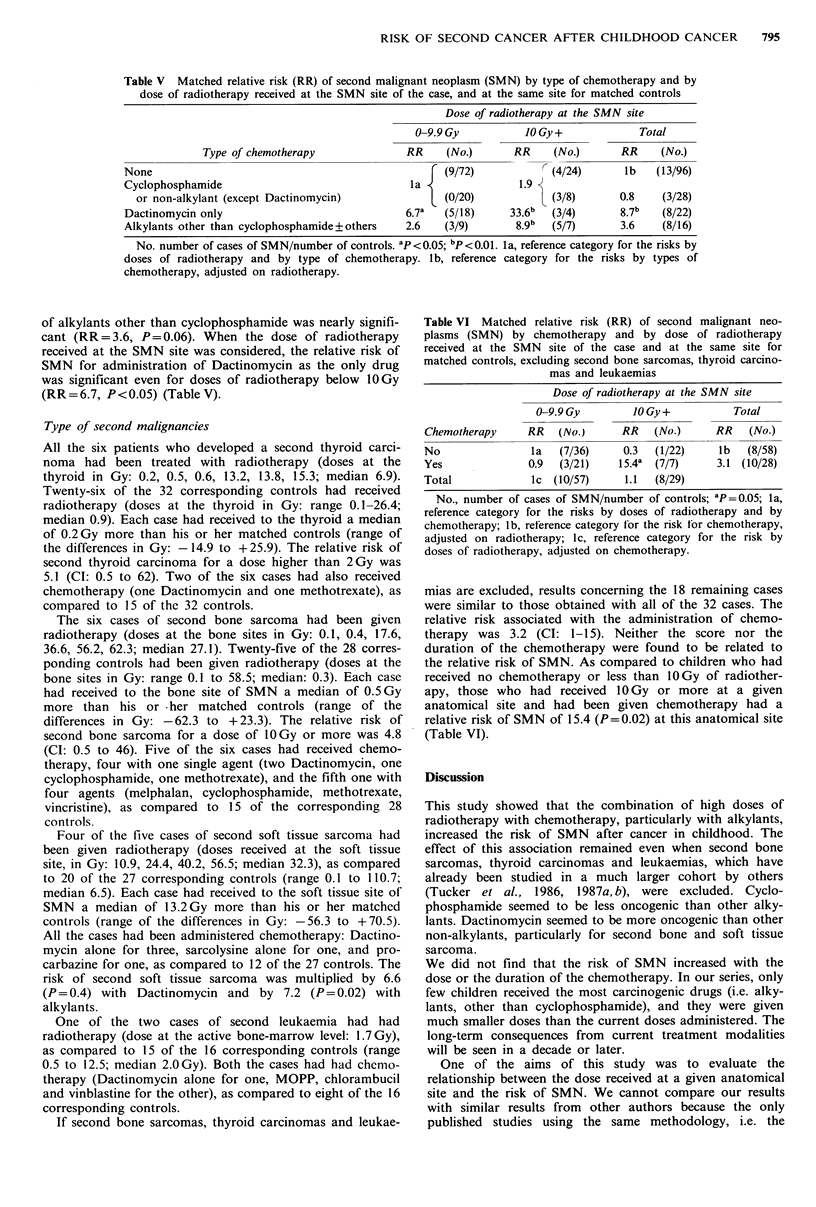
Of a cohort of 634 children treated from 1942 to 1969 at the Gustave Roussy Institute for a first cancer and alive 5 years after treatment, 32 later developed second malignant neoplasms (SMN). A case-control study was performed to determine the relationship between the dose of radiotherapy received on a given anatomical site for the treatment of a first cancer, and the risk of SMN development at the same anatomical site. Another aim of the study was to analyse the effects of the association of radiotherapy with chemotherapy on the risk of SMN. The 32 cases of second malignant neoplasms were individually matched with one to nine patients of the cohort (a total of 162) who did not develop a SMN after a first cancer, matching on age, sex, type of first cancer and follow-up duration. The doses of radiotherapy delivered for the treatment of the first cancer were retrospectively estimated at the 26 anatomical sites of SMN. When the SMN was a leukaemia, the mean active bone-marrow dose was estimated as a weighted mean of the doses received by 20 bone sites. As compared to anatomical sites in children who had not received radiotherapy, the sites which had received 50 Gy or more had a relative risk of SMN of 5.8 (P less than 0.05). When taking into account the dose received at the site of the SMN, neither the number of fractions nor the type of radiations were related to the risk of SMN. Children who had received chemotherapy had a relative risk of SMN of 2.7 (95% CI: 1.2-6.4), adjusted for the dose of radiotherapy, as compared to those who had not. The relative risk of SMN did not vary with the dose nor the duration of the chemotherapy. Dactinomycin was found to increase the relative risk of second soft tissue and bone sarcomas. Cyclophosphamide was found to be less carcinogenic than the other alkylants. The relative risk of SMN was equal to 2.0 (n.s.) after radiotherapy of more than 25 Gy, to 4.4 (n.s.) after chemotherapy, and to 21.4 (P less than 0.01) after the combination of these two treatments modalities, as compared to patients treated by surgery alone. This study suggests that the oncogenic effect of radiations might be increased by chemotherapy, and that the combination of the two treatment modalities might be one of the major factors responsible for overall risk of SMN.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuzick J., Erskine S., Edelman D., Galton D. A. A comparison of the incidence of the myelodysplastic syndrome and acute myeloid leukaemia following melphalan and cyclophosphamide treatment for myelomatosis. A report to the Medical Research Council's working party on leukaemia in adults. Br J Cancer. 1987 May;55(5):523–529. doi: 10.1038/bjc.1987.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angio G. J., Meadows A., Miké V., Harris C., Evans A., Jaffe N., Newton W., Schweisguth O., Sutow W., Morris-Jones P. Decreased risk of radiation-associated second malignant neoplasms in actinomycin-D-treated patients. Cancer. 1976 Feb;37(2 Suppl):1177–1185. doi: 10.1002/1097-0142(197602)37:2+<1177::aid-cncr2820370829>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Greene M. H., Harris E. L., Gershenson D. M., Malkasian G. D., Jr, Melton L. J., 3rd, Dembo A. J., Bennett J. M., Moloney W. C., Boice J. D., Jr Melphalan may be a more potent leukemogen than cyclophosphamide. Ann Intern Med. 1986 Sep;105(3):360–367. doi: 10.7326/0003-4819-105-3-360. [DOI] [PubMed] [Google Scholar]
- Hawkins M. M., Draper G. J., Kingston J. E. Incidence of second primary tumours among childhood cancer survivors. Br J Cancer. 1987 Sep;56(3):339–347. doi: 10.1038/bjc.1987.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M. M. Second primary tumours among survivors of childhood cancer treated with anticancer drugs. IARC Sci Publ. 1986;(78):231–252. [PubMed] [Google Scholar]
- Potish R. A., Dehner L. P., Haselow R. E., Kim T. H., Levitt S. H., Nesbit M. The incidence of second neoplasms following megavoltage radiation for pediatric tumors. Cancer. 1985 Oct 1;56(7):1534–1537. doi: 10.1002/1097-0142(19851001)56:7<1534::aid-cncr2820560711>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Sieber S. M., Correa P., Dalgard D. W., Adamson R. H. Carcinogenic and other adverse effects of procarbazine in nonhuman primates. Cancer Res. 1978 Jul;38(7):2125–2134. [PubMed] [Google Scholar]
- Tucker M. A., D'Angio G. J., Boice J. D., Jr, Strong L. C., Li F. P., Stovall M., Stone B. J., Green D. M., Lombardi F., Newton W. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987 Sep 3;317(10):588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]


