Abstract
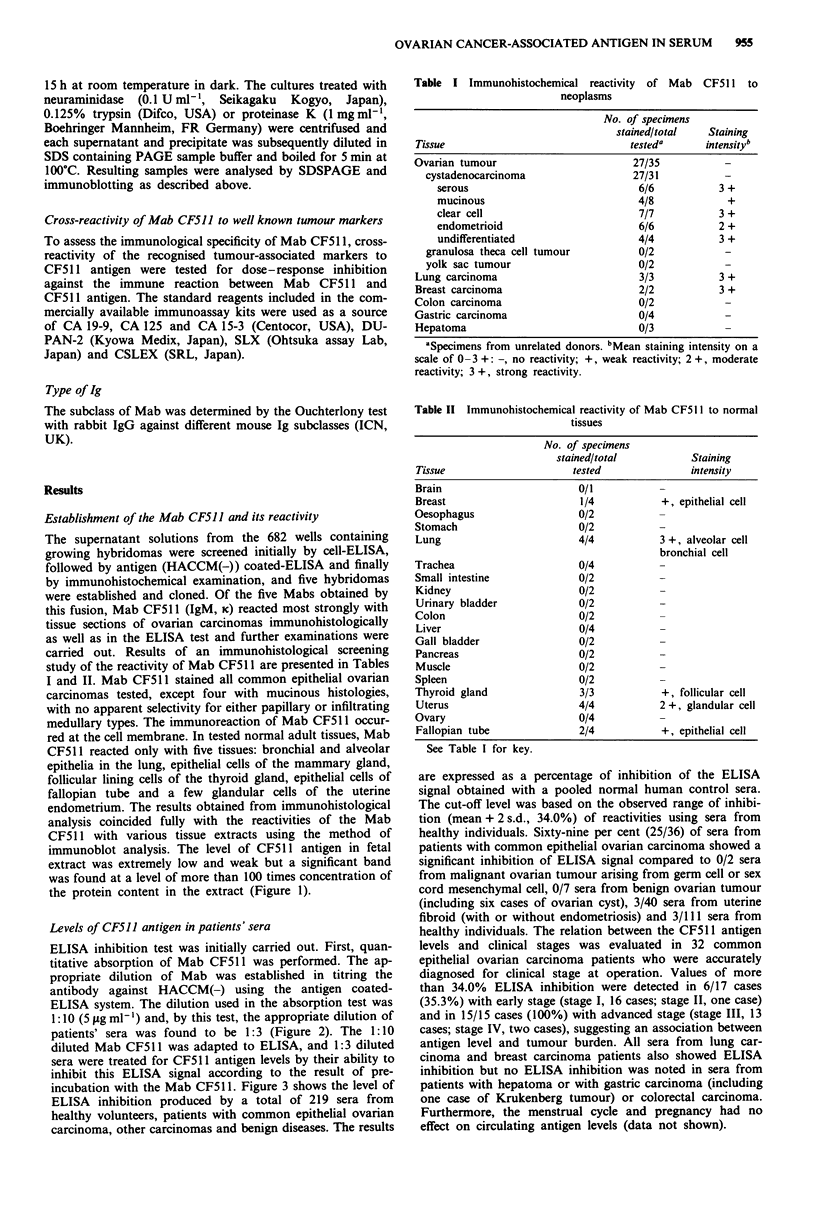
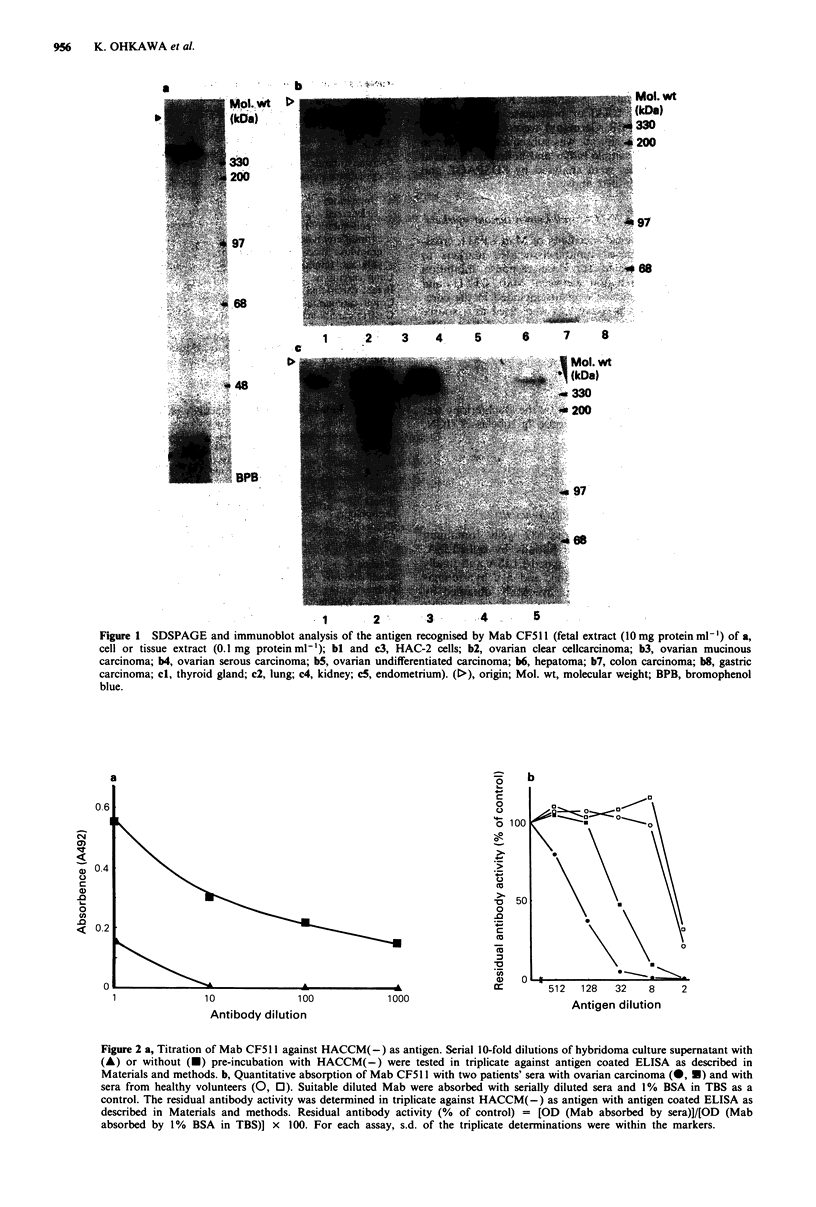
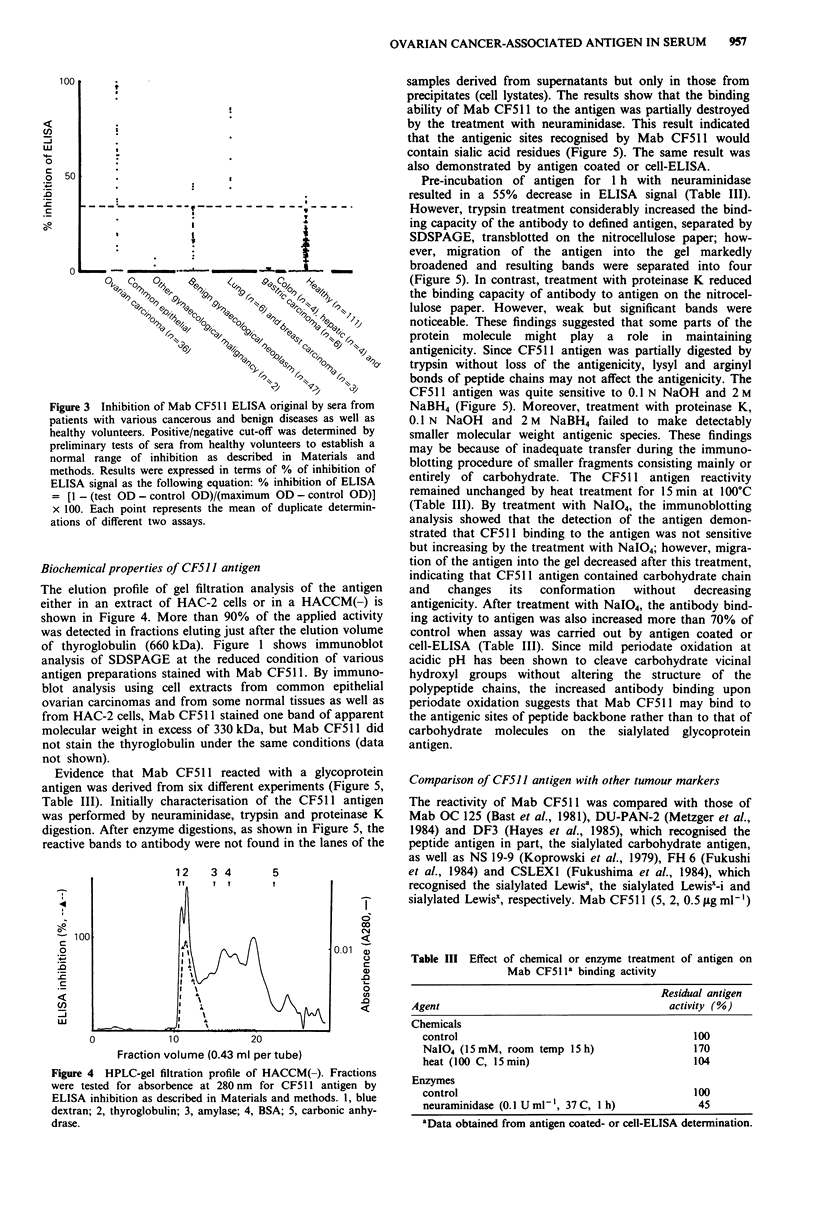
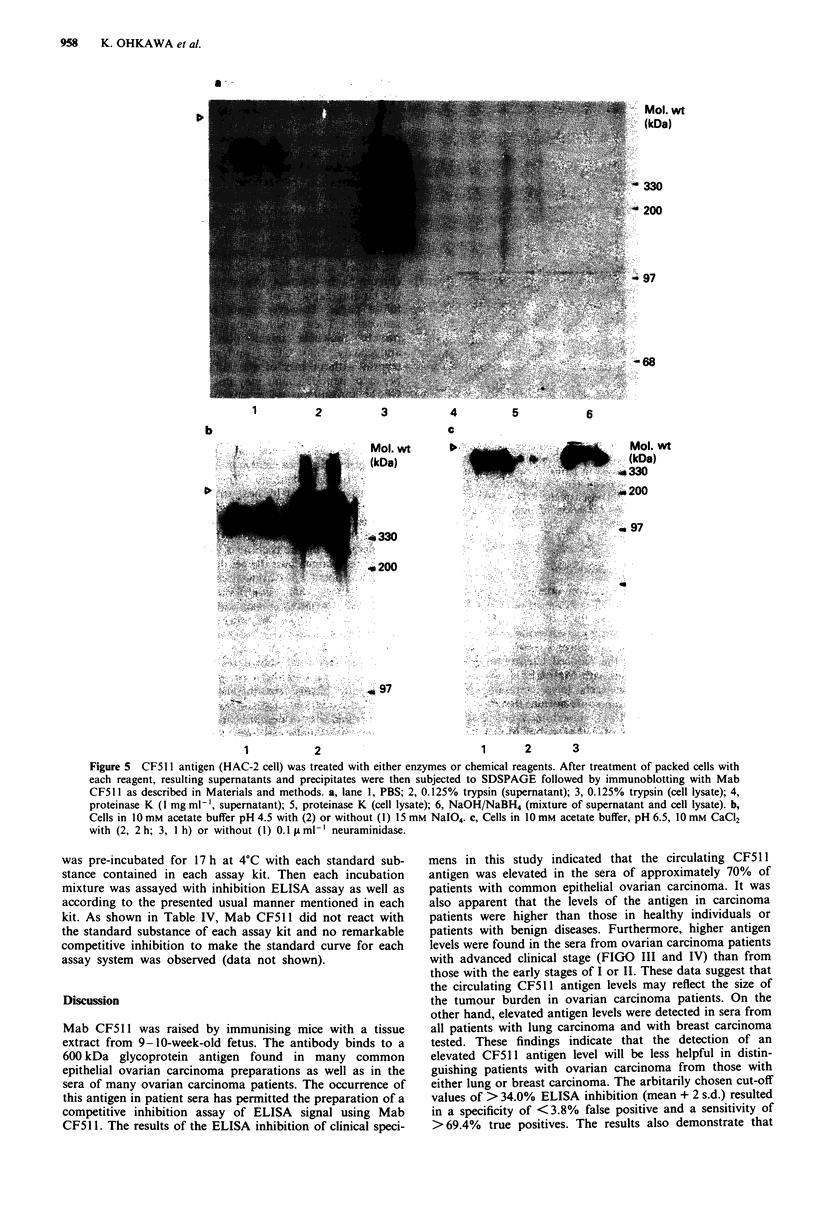
The murine monoclonal antibody (Mab) against human common epithelial ovarian carcinoma, CF511, was generated by immunising mice with human fetal tissue extract from early first trimester, followed by booster injection of an ovarian cancer cell line. Mab CF511 recognised the 600 kDa sialylated glycoprotein as different from previously known tumour associated-marker antigens. Distribution of the Mab CF511-recognised antigen (CF511 antigen) in various tissues and sera was investigated. In immunohistochemical analysis, Mab CF511 reacted strongly with tumour cells of ovarian serous, clear cell, endometrioid and undifferentiated carcinoma and partially with those of mucinous carcinoma. Mab CF511 also reacted with breast carcinoma as well as lung carcinoma. In normal tissues, Mab CF511 cross-reacted with only five tissues, namely lung, breast, thyroid gland, fallopian tube and uterus. Serum levels of CF511 antigen were tested by ELISA inhibition using Mab CF511. This assay showed the circulating CF511 antigen levels to be elevated in 25 of 36 sera from patients with various clinical stages of common epithelial ovarian carcinoma compared to three of 47 and three of 111 sera from patients with other benign gynaecological diseases, including ovarian cysts, uterine fibroids with or without endometriosis and normal healthy subjects, respectively. For the relation between antigen levels and clinical stages of common epithelial ovarian carcinoma, greater than 34.0% ELISA inhibition was detected in 100% of patients with advanced stages (FIGO III and IV) compared with in 35.3% with early stages (FIGO I and II) patients. While patients with breast carcinoma (100%) and lung carcinoma (100%) also had elevated circulating CF511 antigen levels, patients with hepatoma, colorectal carcinoma and gastric carcinoma had no detectable elevation of antigen. Although the test showed a high degree of specificity, the detection of an elevated CF511 antigen level would not be so helpful in distinguishing patients with ovarian carcinoma from those with either breast carcinoma or lung carcinoma. These data suggest that CF511 antigen is a useful new ovarian tumour marker for diagnosis and management of the disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bast R. C., Jr, Feeney M., Lazarus H., Nadler L. M., Colvin R. B., Knapp R. C. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981 Nov;68(5):1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bast R. C., Jr, Klug T. L., St John E., Jenison E., Niloff J. M., Lazarus H., Berkowitz R. S., Leavitt T., Griffiths C. T., Parker L. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983 Oct 13;309(15):883–887. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M., Chatterjee S. K., Barlow J. J., Fuji H. Monoclonal antibodies recognizing tumor-associated antigen of human ovarian mucinous cystadenocarcinomas. Cancer Res. 1982 May;42(5):1650–1654. [PubMed] [Google Scholar]
- Bray K. R., Koda J. E., Gaur P. K. Serum levels and biochemical characteristics of cancer-associated antigen CA-549, a circulating breast cancer marker. Cancer Res. 1987 Nov 15;47(22):5853–5860. [PubMed] [Google Scholar]
- Croghan G. A., Wingate M. B., Gamarra M., Johnson E., Chu T. M., Allen H., Valenzuela L., Tsukada Y., Papsidero L. D. Reactivity of monoclonal antibody F36/22 with human ovarian adenocarcinomas. Cancer Res. 1984 May;44(5):1954–1962. [PubMed] [Google Scholar]
- Davis H. M., Zurawski V. R., Jr, Bast R. C., Jr, Klug T. L. Characterization of the CA 125 antigen associated with human epithelial ovarian carcinomas. Cancer Res. 1986 Dec;46(12 Pt 1):6143–6148. [PubMed] [Google Scholar]
- Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985 Mar 7;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- Friedman E. L., Hayes D. F., Kufe D. W. Reactivity of monoclonal antibody DF3 with a high molecular weight antigen expressed in human ovarian carcinomas. Cancer Res. 1986 Oct;46(10):5189–5194. [PubMed] [Google Scholar]
- Fukushi Y., Nudelman E., Levery S. B., Hakomori S., Rauvala H. Novel fucolipids accumulating in human adenocarcinoma. III. A hybridoma antibody (FH6) defining a human cancer-associated difucoganglioside (VI3NeuAcV3III3Fuc2nLc6). J Biol Chem. 1984 Aug 25;259(16):10511–10517. [PubMed] [Google Scholar]
- Fukushima K., Hirota M., Terasaki P. I., Wakisaka A., Togashi H., Chia D., Suyama N., Fukushi Y., Nudelman E., Hakomori S. Characterization of sialosylated Lewisx as a new tumor-associated antigen. Cancer Res. 1984 Nov;44(11):5279–5285. [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Role of sialic acid in the mobility of membrane proteins containing O-linked oligosaccharides on polyacrylamide gel electrophoresis in sodium dodecyl sulfate. Eur J Biochem. 1982 Mar 1;122(3):581–586. doi: 10.1111/j.1432-1033.1982.tb06478.x. [DOI] [PubMed] [Google Scholar]
- Hayes D. F., Sekine H., Ohno T., Abe M., Keefe K., Kufe D. W. Use of a murine monoclonal antibody for detection of circulating plasma DF3 antigen levels in breast cancer patients. J Clin Invest. 1985 May;75(5):1671–1678. doi: 10.1172/JCI111875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V. G., Schlom J., Paterson A. J., Bennett J., Magnani J. L., Colcher D. Analysis of a human tumor-associated glycoprotein (TAG-72) identified by monoclonal antibody B72.3. Cancer Res. 1986 Feb;46(2):850–857. [PubMed] [Google Scholar]
- Kabawat S. E., Bast R. C., Jr, Bhan A. K., Welch W. R., Knapp R. C., Colvin R. B. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Pathol. 1983;2(3):275–285. doi: 10.1097/00004347-198303000-00005. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Steplewski Z., Mitchell K., Herlyn M., Herlyn D., Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979 Nov;5(6):957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Masuho Y., Zalutsky M., Knapp R. C., Bast R. C., Jr Interaction of monoclonal antibodies with cell surface antigens of human ovarian carcinomas. Cancer Res. 1984 Jul;44(7):2813–2819. [PubMed] [Google Scholar]
- Matsuoka Y., Nakashima T., Endo K., Yoshida T., Kunimatsu M., Sakahara H., Koizumi M., Nakagawa T., Yamaguchi N., Torizuka K. Recognition of ovarian cancer antigen CA125 by murine monoclonal antibody produced by immunization of lung cancer cells. Cancer Res. 1987 Dec 1;47(23):6335–6340. [PubMed] [Google Scholar]
- Mattes M. J., Cordon-Cardo C., Lewis J. L., Jr, Old L. J., Lloyd K. O. Cell surface antigens of human ovarian and endometrial carcinoma defined by mouse monoclonal antibodies. Proc Natl Acad Sci U S A. 1984 Jan;81(2):568–572. doi: 10.1073/pnas.81.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattes M. J., Look K., Furukawa K., Pierce V. K., Old L. J., Lewis J. L., Jr, Lloyd K. O. Mouse monoclonal antibodies to human epithelial differentiation antigens expressed on the surface of ovarian carcinoma ascites cells. Cancer Res. 1987 Dec 15;47(24 Pt 1):6741–6750. [PubMed] [Google Scholar]
- Metzgar R. S., Rodriguez N., Finn O. J., Lan M. S., Daasch V. N., Fernsten P. D., Meyers W. C., Sindelar W. F., Sandler R. S., Seigler H. F. Detection of a pancreatic cancer-associated antigen (DU-PAN-2 antigen) in serum and ascites of patients with adenocarcinoma. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5242–5246. doi: 10.1073/pnas.81.16.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotti S., Aguanno S., Canevari S., Diotti A., Orlandi R., Sonnino S., Colnaghi M. I. Biochemical analysis of human ovarian cancer-associated antigens defined by murine monoclonal antibodies. Cancer Res. 1985 Feb;45(2):826–832. [PubMed] [Google Scholar]
- Miotti S., Canevari S., Ménard S., Mezzanzanica D., Porro G., Pupa S. M., Regazzoni M., Tagliabue E., Colnaghi M. I. Characterization of human ovarian carcinoma-associated antigens defined by novel monoclonal antibodies with tumor-restricted specificity. Int J Cancer. 1987 Mar 15;39(3):297–303. doi: 10.1002/ijc.2910390306. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982 Sep;42(9):3858–3863. [PubMed] [Google Scholar]
- Nakagawa S., Tsuji Y., Masuda N., Nishiura H., Isojima S. Establishment of a murine monoclonal antibody against human clear cell carcinoma and analysis of the corresponding antigen. Gynecol Oncol. 1987 Nov;28(3):318–329. doi: 10.1016/0090-8258(87)90179-x. [DOI] [PubMed] [Google Scholar]
- Niloff J. M., Knapp R. C., Schaetzl E., Reynolds C., Bast R. C., Jr CA125 antigen levels in obstetric and gynecologic patients. Obstet Gynecol. 1984 Nov;64(5):703–707. [PubMed] [Google Scholar]
- Nouwen E. J., Pollet D. E., Eerdekens M. W., Hendrix P. G., Briers T. W., De Broe M. E. Immunohistochemical localization of placental alkaline phosphatase, carcinoembryonic antigen, and cancer antigen 125 in normal and neoplastic human lung. Cancer Res. 1986 Feb;46(2):866–876. [PubMed] [Google Scholar]
- O'Brien T. J., Hardin J. W., Bannon G. A., Norris J. S., Quirk J. G., Jr CA 125 antigen in human amniotic fluid and fetal membranes. Am J Obstet Gynecol. 1986 Jul;155(1):50–55. doi: 10.1016/0002-9378(86)90076-1. [DOI] [PubMed] [Google Scholar]
- Sakakibara K., Ueda R., Ohta M., Nakashima N., Tomoda Y., Takahashi T. Three novel mouse monoclonal antibodies, OM-A, OM-B, and OM-C, reactive with mucinous type ovarian tumors. Cancer Res. 1988 Aug 15;48(16):4639–4645. [PubMed] [Google Scholar]
- Sekine H., Ohno T., Kufe D. W. Purification and characterization of a high molecular weight glycoprotein detectable in human milk and breast carcinomas. J Immunol. 1985 Nov;135(5):3610–3615. [PubMed] [Google Scholar]
- Tagliabue E., Mènard S., Della Torre G., Barbanti P., Mariani-Costantini R., Porro G., Colnaghi M. I. Generation of monoclonal antibodies reacting with human epithelial ovarian cancer. Cancer Res. 1985 Jan;45(1):379–385. [PubMed] [Google Scholar]
- Thor A., Gorstein F., Ohuchi N., Szpak C. A., Johnston W. W., Schlom J. Tumor-associated glycoprotein (TAG-72) in ovarian carcinomas defined by monoclonal antibody B72.3. J Natl Cancer Inst. 1986 Jun;76(6):995–1006. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji Y., Suzuki T., Nishiura H., Takemura T., Isojima S. Identification of two different surface epitopes of human ovarian epithelial carcinomas by monoclonal antibodies. Cancer Res. 1985 May;45(5):2358–2362. [PubMed] [Google Scholar]