Abstract
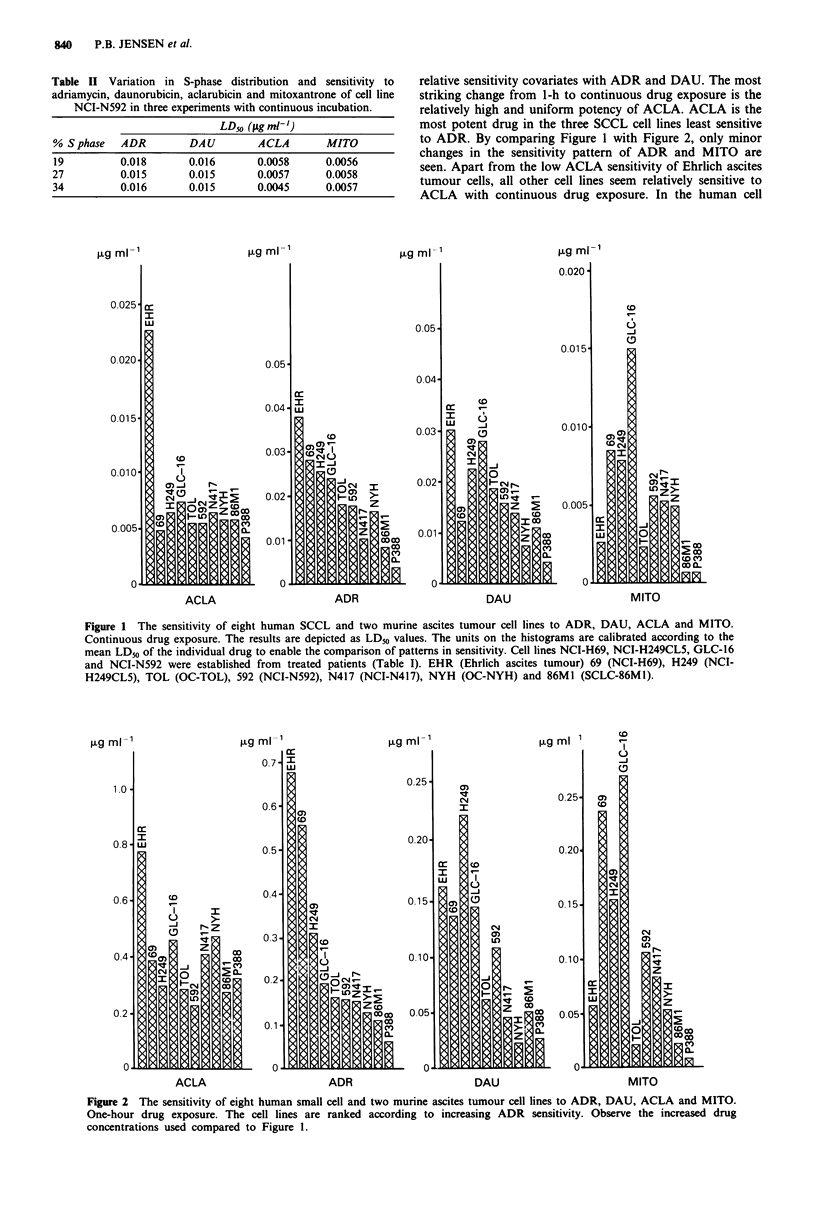
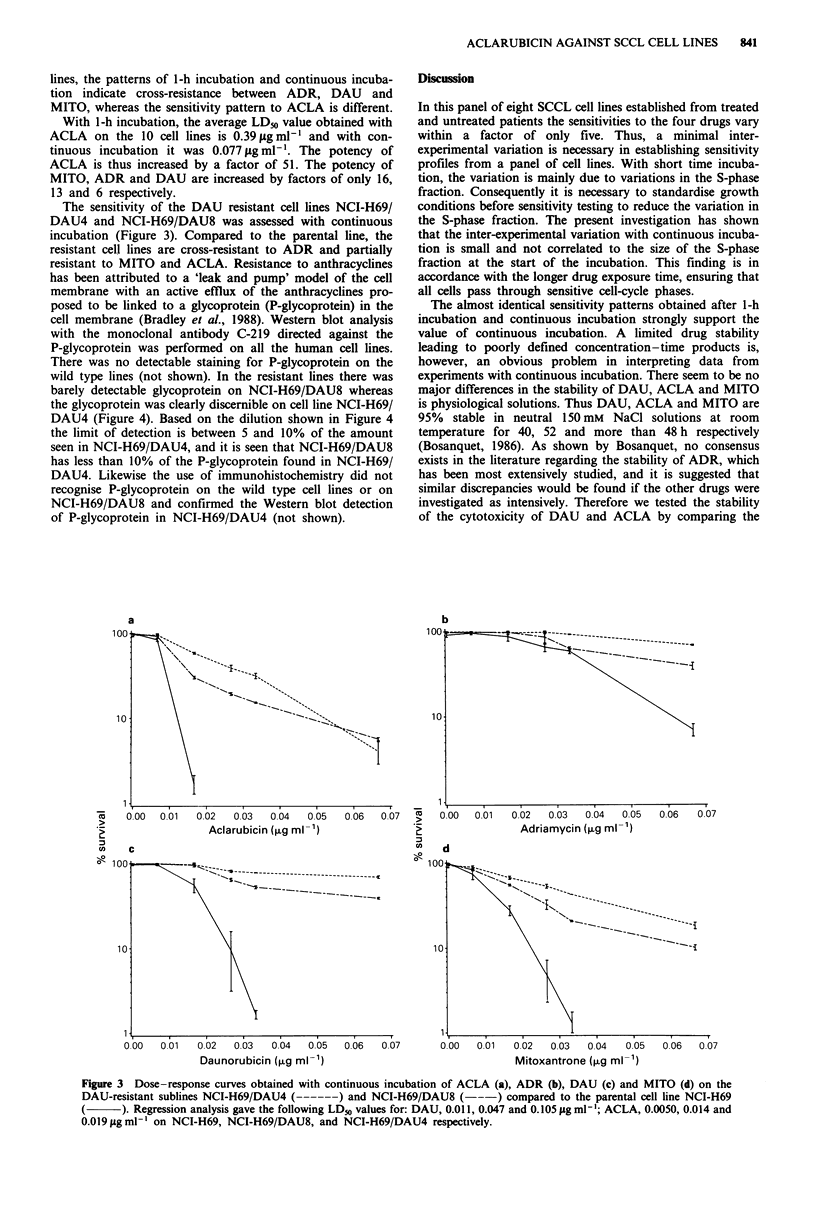
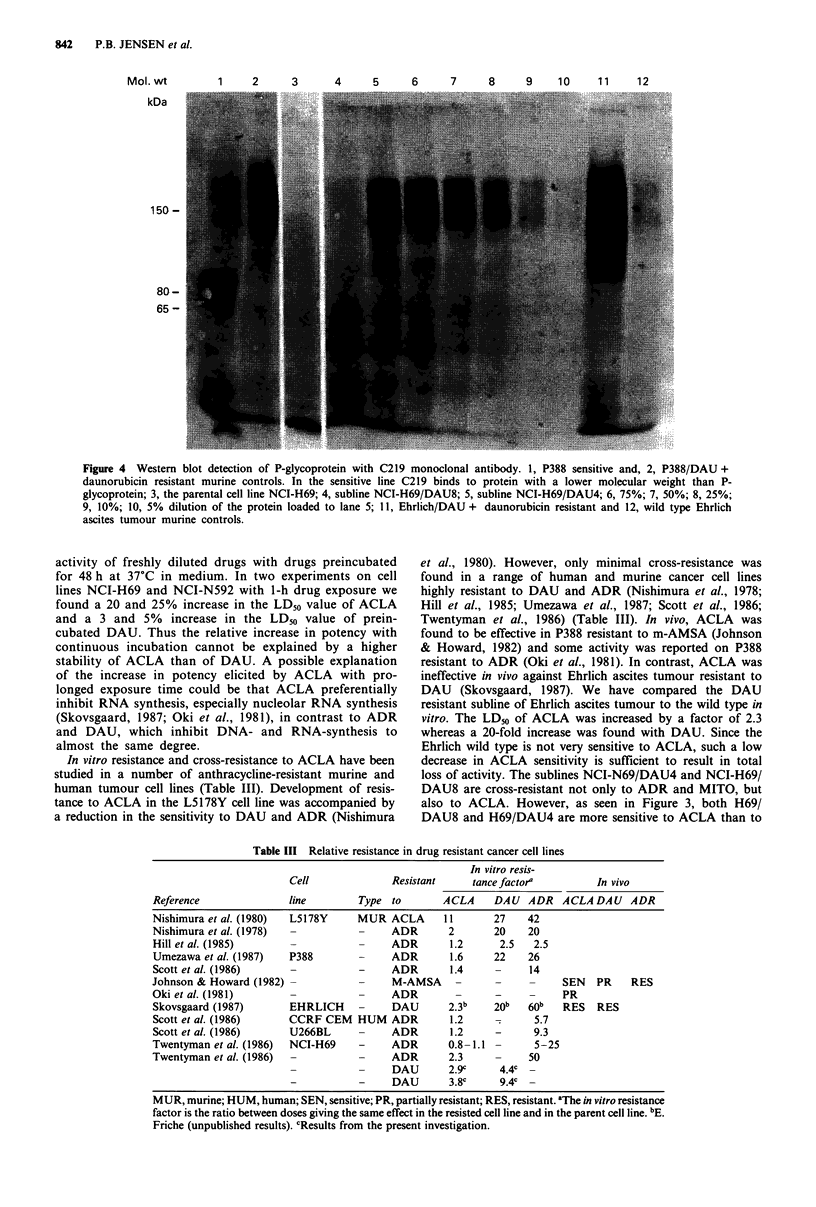
The sensitivity of eight cell lines established from treated and untreated patients with small cell carcinoma of the lung (SCCL) was tested in the clonogenic assay with 1 h and continuous exposure to aclarubicin (ACLA), adriamycin (ADR), daunorubicin (DAU) and mitoxantrone (MITO). The sensitivity to ADR, DAU and MITO covariated, and varied with a factor of five. The sensitivity to ACLA was independent of the sensitivity to ADR and varied only within a factor of two. Only ACLA showed pronounced increased potency with continuous incubation, and ACLA was the most potent drug in the three cell lines least sensitive to ADR. Two resistant cell lines were selected by treating NCI-H69 in vitro with DAU. One cell line (9-fold resistant to DAU) expressed large amounts of P-glycoprotein, the other cell line (4-fold resistant to DAU) had barely detectable glycoprotein. Both lines acquired resistance to ADR, ACLA and MITO. The cross-resistance to ACLA and MITO was only partial and ACLA was still the most potent drug on these lines. The sensitivity to ACLA of the cell lines least sensitive to ADR suggest that ACLA partially circumvents mechanisms of multidrug resistance. Together with the pronounced increase in potency with prolonged exposure, these results suggest that ACLA has a mechanism of action different from the 'classical' anthracyclines. In this context mitoxantrone is more similar to the classical anthracyclines although its structure is more dissimilar.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aabo K., Mortensen S. A., Skovsgaard T., Gymoese E. Intermittent high-dose aclarubicin in patients with advanced cancer: a phase I study with special reference to cardiac toxicity. Cancer Treat Rep. 1983 Mar;67(3):281–282. [PubMed] [Google Scholar]
- Abeloff M. D., Finkelstein D. M., Chang A. Y., Camacho F. J., Creech R. H., Ettinger D. S. Phase II study of aclarubicin and diaziquone in the treatment of advanced small cell bronchogenic carcinoma (EST 4581): an Eastern Cooperative Oncology Group Study. Cancer Treat Rep. 1985 Apr;69(4):451–452. [PubMed] [Google Scholar]
- Bosanquet A. G. Stability of solutions of antineoplastic agents during preparation and storage for in vitro assays. II. Assay methods, adriamycin and the other antitumour antibiotics. Cancer Chemother Pharmacol. 1986;17(1):1–10. doi: 10.1007/BF00299858. [DOI] [PubMed] [Google Scholar]
- Bradley G., Juranka P. F., Ling V. Mechanism of multidrug resistance. Biochim Biophys Acta. 1988 Aug 3;948(1):87–128. doi: 10.1016/0304-419x(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Carney D. N., Gazdar A. F., Bepler G., Guccion J. G., Marangos P. J., Moody T. W., Zweig M. H., Minna J. D. Establishment and identification of small cell lung cancer cell lines having classic and variant features. Cancer Res. 1985 Jun;45(6):2913–2923. [PubMed] [Google Scholar]
- Hill B. T., Dennis L. Y., Li X. T., Whelan R. D. Identification of anthracycline analogues with enhanced cytotoxicity and lack of cross-resistance to adriamycin using a series of mammalian cell lines in vitro. Cancer Chemother Pharmacol. 1985;14(3):194–201. doi: 10.1007/BF00258115. [DOI] [PubMed] [Google Scholar]
- Hill B. T., Hosking L. K., Shellard S. A., Whelan R. D. Comparative effectiveness of mitoxantrone and doxorubicin in overcoming experimentally induced drug resistance in murine and human tumour cell lines in vitro. Cancer Chemother Pharmacol. 1989;23(3):140–144. doi: 10.1007/BF00267944. [DOI] [PubMed] [Google Scholar]
- Johnson R. K., Howard W. S. Development and cross-resistance characteristics of a subline of P388 leukemia resistant to 4'-(9-acridinylamino)-methanesulfon-m-anisidide. Eur J Cancer Clin Oncol. 1982 May;18(5):479–487. doi: 10.1016/0277-5379(82)90117-1. [DOI] [PubMed] [Google Scholar]
- Kartner N., Evernden-Porelle D., Bradley G., Ling V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. 1985 Aug 29-Sep 4Nature. 316(6031):820–823. doi: 10.1038/316820a0. [DOI] [PubMed] [Google Scholar]
- Kerpel-Fronius S., Gyergyay F., Hindy I., Decker A., Sawinsky I., Fäller K., Mechl Z., Nekulova M., Kolaric K., Tomek R. Phase I-II trial of aclacinomycin A given in a four-consecutive-day schedule to patients with solid tumours. A South-East European Oncology Group (SEEOG) Study. Oncology. 1987;44(3):159–163. doi: 10.1159/000226469. [DOI] [PubMed] [Google Scholar]
- Kramer B. S., Birch R., Gockerman J. P., Greco A., Prestridge K. Phase II evaluation of aclarubicin in lung cancer: a Southeastern Cancer Study Group Trial. Cancer Treat Rep. 1986 Jun;70(6):803–804. [PubMed] [Google Scholar]
- Machover D., Gastiaburu J., Delgado M., Goldschmidt E., Hulhoven R., Misset J. L., de Vassal F., Tapiero H., Ribaud P., Schwarzenberg L. Phase I-II study of aclarubicin for treatment of acute myeloid leukemia. Cancer Treat Rep. 1984 Jun;68(6):881–886. [PubMed] [Google Scholar]
- Nishimura T., Muto K., Tanaka N. Drug sensitivity of an adriamycin-resistant mutant subline of mouse lymphoblastoma L5178Y cells. J Antibiot (Tokyo) 1978 May;31(5):493–495. doi: 10.7164/antibiotics.31.493. [DOI] [PubMed] [Google Scholar]
- Oki T., Matsuzawa Y., Yoshimoto A., Numata K., Kitamura I. New antitumor antibiotics aclacinomycins A and B. J Antibiot (Tokyo) 1975 Oct;28(10):830–834. doi: 10.7164/antibiotics.28.830. [DOI] [PubMed] [Google Scholar]
- Oki T., Takeuchi T., Oka S., Umezawa H. New anthracycline antibiotic aclacinomycin A: experimental studies and correlations with clinical trials. Recent Results Cancer Res. 1981;76:21–40. doi: 10.1007/978-3-642-81565-2_4. [DOI] [PubMed] [Google Scholar]
- Pedersen-Bjergaard J., Brincker H., Ellegaard J., Drivsholm A., Freund L., Jensen K. B., Jensen M. K., Nissen N. I. Aclarubicin in the treatment of acute nonlymphocytic leukemia refractory to treatment with daunorubicin and cytarabine: a phase II trial. Cancer Treat Rep. 1984 Oct;68(10):1233–1238. [PubMed] [Google Scholar]
- Roed H., Christensen I. B., Vindeløv L. L., Spang-Thomsen M., Hansen H. H. Inter-experiment variation and dependence on culture conditions in assaying the chemosensitivity of human small cell lung cancer cell lines. Eur J Cancer Clin Oncol. 1987 Feb;23(2):177–186. doi: 10.1016/0277-5379(87)90012-5. [DOI] [PubMed] [Google Scholar]
- Scott C. A., Westmacott D., Broadhurst M. J., Thomas G. J., Hall M. J. 9-alkyl anthracyclines. Absence of cross-resistance to adriamycin in human and murine cell cultures. Br J Cancer. 1986 May;53(5):595–600. doi: 10.1038/bjc.1986.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovsgaard T. Pharmacodynamic aspects of aclarubicin with special reference to daunorubicin and doxorubicin. Eur J Haematol Suppl. 1987;47:7–20. doi: 10.1111/j.1600-0609.1987.tb00018.x. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., Wright K. A., Bleehen N. M. Derivation and preliminary characterisation of adriamycin resistant lines of human lung cancer cells. Br J Cancer. 1986 Apr;53(4):529–537. doi: 10.1038/bjc.1986.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa K., Kunimoto S., Takeuchi T. Experimental studies of new anthracyclines: aclacinomycin, THP-adriamycin and ditrisarubicins. Biomed Pharmacother. 1987;41(5):206–213. [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Keiding N., Spang-Thomsen M., Nissen N. I. Long-term storage of samples for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):317–322. doi: 10.1002/cyto.990030502. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Warrell R. P., Jr, Arlin Z. A., Kempin S. J., Young C. W. Phase I--II evaluation of a new anthracycline antibiotic, aclacinomycin A, in adults with refractory leukemia. Cancer Treat Rep. 1982 Aug;66(8):1619–1623. [PubMed] [Google Scholar]
- Woolley P. V., 3rd, Ayoob M. J., Levenson S. M., Smith F. P. A phase I clinical trial of aclacinomycin A administered on a five-consecutive-day schedule. J Clin Pharmacol. 1982 Aug-Sep;22(8-9):359–365. doi: 10.1002/j.1552-4604.1982.tb02686.x. [DOI] [PubMed] [Google Scholar]
- de Leij L., Postmus P. E., Buys C. H., Elema J. D., Ramaekers F., Poppema S., Brouwer M., van der Veen A. Y., Mesander G., The T. H. Characterization of three new variant type cell lines derived from small cell carcinoma of the lung. Cancer Res. 1985 Dec;45(12 Pt 1):6024–6033. [PubMed] [Google Scholar]




