Abstract
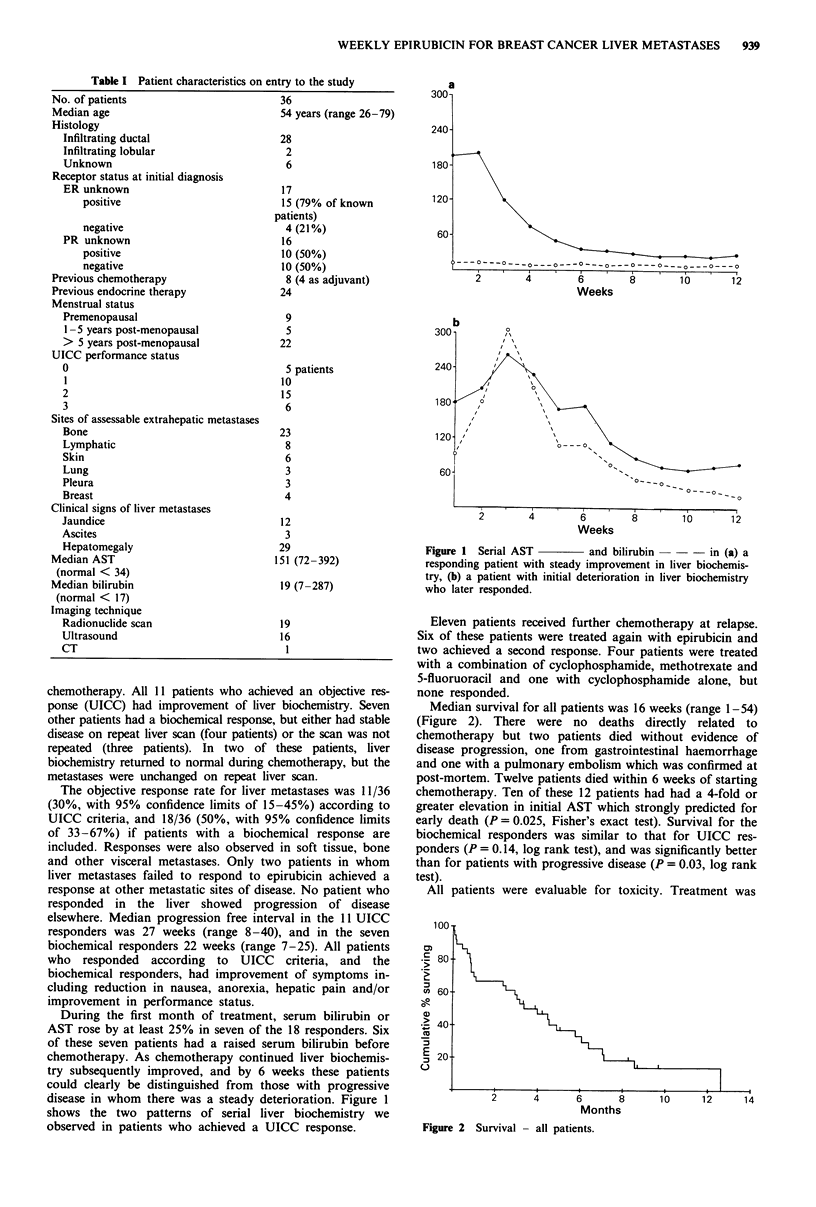
Thirty-six consecutive patients with breast cancer and liver metastases with abnormal liver biochemistry were treated with epirubicin 25 mg m-2 i.v. weekly. No dose modification was made for abnormal liver biochemistry, but dose intensity was adjusted by delaying treatment according to myelosuppression. The UICC overall response rate according to UICC criteria was 11/36 (30%) and median response duration was 27 weeks. Liver biochemistry improved in a further seven patients. Treatment was well tolerated. Epirubicin given in this way is effective in patients with breast cancer and liver metastases. An initial deterioration in liver biochemistry may occur before there is a response to epirubicin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagley C. M., Jr, Bostick F. W., DeVita V. T., Jr Clinical pharmacology of cyclophosphamide. Cancer Res. 1973 Feb;33(2):226–233. [PubMed] [Google Scholar]
- Benjamin R. S., Wiernik P. H., Bachur N. R. Adriamycin chemotherapy--efficacy, safety, and pharmacologic basis of an intermittent single high-dosage schedule. Cancer. 1974 Jan;33(1):19–27. doi: 10.1002/1097-0142(197401)33:1<19::aid-cncr2820330107>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Brambilla C., Rossi A., Bonfante V., Ferrari L., Villani F., Crippa F., Bonadonna G. Phase II study of doxorubicin versus epirubicin in advanced breast cancer. Cancer Treat Rep. 1986 Feb;70(2):261–266. [PubMed] [Google Scholar]
- Camaggi C. M., Comparsi R., Strocchi E., Testoni F., Angelelli B., Pannuti F. Epirubicin and doxorubicin comparative metabolism and pharmacokinetics. A cross-over study. Cancer Chemother Pharmacol. 1988;21(3):221–228. doi: 10.1007/BF00262774. [DOI] [PubMed] [Google Scholar]
- Camaggi C. M., Strocchi E., Tamassia V., Martoni A., Giovannini M., Lafelice G., Canova N., Marraro D., Martini A., Pannuti F. Pharmacokinetic studies of 4'-epi-doxorubicin in cancer patients with normal and impaired renal function and with hepatic metastases. Cancer Treat Rep. 1982 Oct;66(10):1819–1824. [PubMed] [Google Scholar]
- Carmo-Pereira J., Costa F. O., Henriques E., Godinho F., Cantinho-Lopes M. G., Sales-Luis A., Rubens R. D. A comparison of two doses of adriamycin in the primary chemotherapy of disseminated breast carcinoma. Br J Cancer. 1987 Oct;56(4):471–473. doi: 10.1038/bjc.1987.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski R. T., Irwin L. E., Pugh R. P., Sadoff L., Hestorff R., Wiener J. M., Bateman J. R. Survival of patients with metastatic breast cancer treated with either combination or sequential chemotherapy. Cancer Res. 1979 Nov;39(11):4503–4506. [PubMed] [Google Scholar]
- Coleman R. E., Rubens R. D. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987 Jan;55(1):61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fentiman I. S., Lavelle M. A., Caplan D., Miller N., Millis R. R., Hayward J. L. The significance of supraclavicular fossa node recurrence after radical mastectomy. Cancer. 1986 Mar 1;57(5):908–910. doi: 10.1002/1097-0142(19860301)57:5<908::aid-cncr2820570504>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Kemeny N. The systemic chemotherapy of hepatic metastases. Semin Oncol. 1983 Jun;10(2):148–158. [PubMed] [Google Scholar]
- Kennealey G. T., Boston B., Mitchell M. S., Knobf M. K., Bobrow S. N., Pezzimenti J. F., Lawrence R., Bertino J. R. Combination chemotherapy for advanced breast cancer: two regimens containing adriamycin. Cancer. 1978 Jul;42(1):27–33. doi: 10.1002/1097-0142(197807)42:1<27::aid-cncr2820420105>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Larroquette C. A., Hortobagyi G. N., Buzdar A. U., Holmes F. A. Subclinical hepatic toxicity during combination chemotherapy for breast cancer. JAMA. 1986 Dec 5;256(21):2988–2990. [PubMed] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981 Jan 1;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Preiss R., Matthias M., Sohr R., Brockmann B., Hüller H. Pharmacokinetics of adriamycin, adriamycinol, and antipyrine in patients with moderate tumor involvement of the liver. J Cancer Res Clin Oncol. 1987;113(6):593–598. doi: 10.1007/BF00390872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley R. V., Carpenter J., Bartolucci A., Vogel C., Krauss S. A comparison of cyclophosphamide, adriamycin, 5-fluorouracil (CAF) and cyclophosphamide, methotrexate, 5-fluorouracil, vincristine, prednisone (CMFVP) in patients with metastatic breast cancer: a Southeastern Cancer Study Group project. Cancer. 1977 Aug;40(2):625–632. doi: 10.1002/1097-0142(197708)40:2<625::aid-cncr2820400206>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Steiner R., Stewart J. F., Cantwell B. M., Minton M. J., Knight R. K., Rubens R. D. Adriamycin alone or combined with vincristine in the treatment of advanced breast cancer. Eur J Cancer Clin Oncol. 1983 Nov;19(11):1553–1557. doi: 10.1016/0277-5379(83)90085-8. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Okazaki N., Yoshino M., Ohkura H., Miyamoto K., Shimada Y. Phase II trial of mitoxantrone in patients with hepatocellular carcinoma. Eur J Cancer Clin Oncol. 1988 Dec;24(12):1897–1898. doi: 10.1016/0277-5379(88)90104-6. [DOI] [PubMed] [Google Scholar]
- Zinser J. W., Hortobagyi G. N., Buzdar A. U., Smith T. L., Fraschini G. Clinical course of breast cancer patients with liver metastases. J Clin Oncol. 1987 May;5(5):773–782. doi: 10.1200/JCO.1987.5.5.773. [DOI] [PubMed] [Google Scholar]



