Abstract
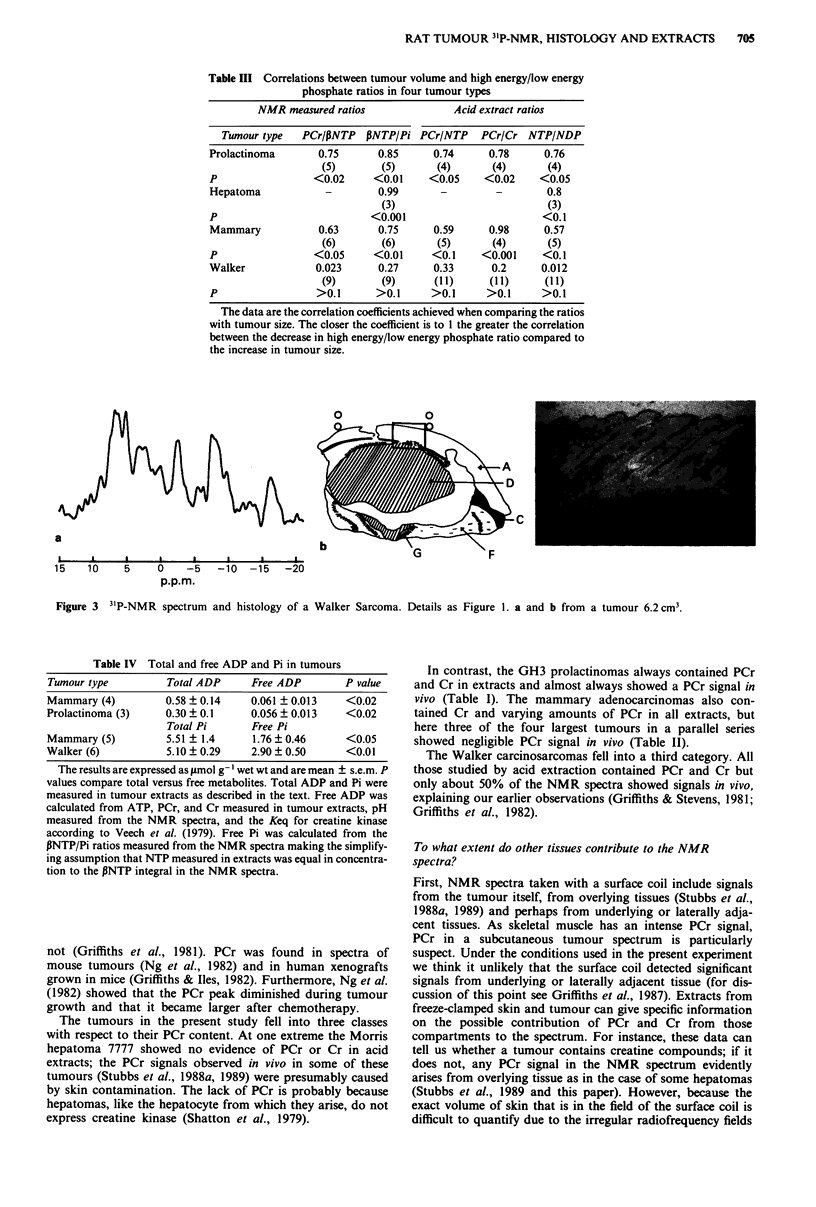
31P-NMP, surface coil spectra of three subcutaneously implanted rat tumours (Morris hepatoma 7777, GH3 prolactinoma, Walker carcinosarcoma) and an N-methyl-N-nitrosourea induced rat mammary adenocarcinoma at different stages of growth were obtained and compared with histological sections taken immediately after NMR acquisitions. Metabolite ratios (phosphocreatine (PCr)/beta nucleoside triphosphate (beta NTP), PCr/Pi, beta NTP/Pi) calculated from the NMR spectra were compared with ratios obtained from acid extracts of tumours of similar size. Measurements of creatine and ADP were also made. Three of the tumours showed positive correlations between increasing tumour size and decreasing metabolite ratios measured both by NMR and in extracts, whereas the Walker carcinosarcoma showed no correlation between size and any parameters measured. Phosphorus metabolite ratios, measured in extracts of skin overlying the tumours, indicated a fall in high energy phosphate when there was histological evidence of skin invasion by the tumour. Surface coil 31P-NMR spectra of subcutaneously grown or induced tumours in the rat represent a slowly changing steady state as the tumour increases in size. We conclude that increasing numbers of hypoxic tumour cells, rather than large areas of necrotic tissue, contribute largely to the NMR spectrum.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman J. J., Grove T. H., Wong G. G., Gadian D. G., Radda G. K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980 Jan 10;283(5743):167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- Adams D. A., DeNardo G. L., DeNardo S. J., Conboy C. B., Bradbury E. M. 31P surface-coil NMR analysis of metabolic status in KHJJ tumors. Magn Reson Med. 1985 Oct;2(5):419–427. doi: 10.1002/mrm.1910020502. [DOI] [PubMed] [Google Scholar]
- ChandraRajan J., Klein L. Determination of inorganic phosphorus in the presence of organic phosphorus and high concentrations of proteins. Anal Biochem. 1976 May 7;72:407–412. doi: 10.1016/0003-2697(76)90548-0. [DOI] [PubMed] [Google Scholar]
- Corbett R. J., Nunnally R. L., Giovanella B. C., Antich P. P. Characterization of the 31P nuclear magnetic resonance spectrum from human melanoma tumors implanted in nude mice. Cancer Res. 1987 Oct 1;47(19):5065–5069. [PubMed] [Google Scholar]
- Evanochko W. T., Ng T. C., Glickson J. D. Application of in vivo NMR spectroscopy to cancer. Magn Reson Med. 1984 Dec;1(4):508–534. doi: 10.1002/mrm.1910010410. [DOI] [PubMed] [Google Scholar]
- Freeman D., Bartlett S., Radda G., Ross B. Energetics of sodium transport in the kidney. Saturation transfer 31P-NMR. Biochim Biophys Acta. 1983 Apr 5;762(2):325–336. doi: 10.1016/0167-4889(83)90087-3. [DOI] [PubMed] [Google Scholar]
- Griffiths J. R., Bhujwalla Z., Coombes R. C., Maxwell R. J., Midwood C. J., Morgan R. J., Nias A. H., Perry P., Prior M., Prysor-Jones R. A. Monitoring cancer therapy by NMR spectroscopy. Ann N Y Acad Sci. 1987;508:183–199. doi: 10.1111/j.1749-6632.1987.tb32904.x. [DOI] [PubMed] [Google Scholar]
- Griffiths J. R., Iles R. A. NMR studies of tumours. Biosci Rep. 1982 Sep;2(9):719–725. doi: 10.1007/BF01114835. [DOI] [PubMed] [Google Scholar]
- Griffiths J. R., Stevens A. N., Iles R. A., Gordon R. E., Shaw D. 31P-NMR investigation of solid tumours in the living rat. Biosci Rep. 1981 Apr;1(4):319–325. doi: 10.1007/BF01114871. [DOI] [PubMed] [Google Scholar]
- Iles R. A., Stevens A. N., Griffiths J. R., Morris P. G. Phosphorylation status of liver by 31P-n.m.r. spectroscopy, and its implications for metabolic control. A comparison of 31P-n.m.r. spectroscopy (in vivo and in vitro) with chemical and enzymic determinations of ATP, ADP and Pi. Biochem J. 1985 Jul 1;229(1):141–151. doi: 10.1042/bj2290141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGilvery R. W., Murray T. W. Calculated equilibria of phosphocreatine and adenosine phosphates during utilization of high energy phosphate by muscle. J Biol Chem. 1974 Sep 25;249(18):5845–5850. [PubMed] [Google Scholar]
- O'Carra P. Biospecific binding to immobilized small ligands in affinity chromatography. Biochem Soc Trans. 1981 Aug;9(4):283–283. doi: 10.1042/bst0090283. [DOI] [PubMed] [Google Scholar]
- Prichard J. W., Alger J. R., Behar K. L., Petroff O. A., Shulman R. G. Cerebral metabolic studies in vivo by 31P NMR. Proc Natl Acad Sci U S A. 1983 May;80(9):2748–2751. doi: 10.1073/pnas.80.9.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy C., Albrand J. P., Benabid A. L., Decorps M., Jacrot M., Riondel J., Foray M. F. In vivo 31P nuclear magnetic resonance studies of T1 and T2 relaxation times in rat brain and in rat brain tumors implanted to nude mice. Magn Reson Med. 1987 Feb;4(2):144–152. doi: 10.1002/mrm.1910040207. [DOI] [PubMed] [Google Scholar]
- Rodrigues L. M., Midwood C. J., Coombes R. C., Stevens A. N., Stubbs M., Griffiths J. R. 31P-nuclear magnetic resonance spectroscopy studies of the response of rat mammary tumors to endocrine therapy. Cancer Res. 1988 Jan 1;48(1):89–93. [PubMed] [Google Scholar]
- Rofstad E. K., DeMuth P., Sutherland R. M. 31P NMR spectroscopy measurements of human ovarian carcinoma xenografts: relationship to tumour volume, growth rate, necrotic fraction and differentiation status. Radiother Oncol. 1988 Aug;12(4):315–326. doi: 10.1016/0167-8140(88)90021-7. [DOI] [PubMed] [Google Scholar]
- Rofstad E. K., Howell R. L., DeMuth P., Ceckler T. L., Sutherland R. M. 31P NMR spectroscopy in vivo of two murine tumor lines with widely different fractions of radiobiologically hypoxic cells. Int J Radiat Biol. 1988 Oct;54(4):635–649. doi: 10.1080/09553008814552071. [DOI] [PubMed] [Google Scholar]
- Ross B. D., Freeman D. M., Chan L. Phosphorus metabolites by NMR. Adv Exp Med Biol. 1984;178:455–464. doi: 10.1007/978-1-4684-4808-5_56. [DOI] [PubMed] [Google Scholar]
- Shatton J. B., Morris H. P., Weinhouse S. Creatine kinase activity and isozyme composition in normal tissues and neoplasms of rats and mice. Cancer Res. 1979 Feb;39(2 Pt 1):492–501. [PubMed] [Google Scholar]
- Shuttlewood R. J., Griffiths J. R. The purine nucleotide profile in mouse, chicken and human dystrophic muscle: an abnormal ratio of inosine plus adenine nucleotides to guanine nucleotides. Clin Sci (Lond) 1982 Jan;62(1):113–115. doi: 10.1042/cs0620113. [DOI] [PubMed] [Google Scholar]
- Steen R. G., Tamargo R. J., McGovern K. A., Rajan S. S., Brem H., Wehrle J. P., Glickson J. D. In vivo 31P nuclear magnetic resonance spectroscopy of subcutaneous 9L gliosarcoma: effects of tumor growth and treatment with 1,3-bis(2-chloroethyl)-1-nitrosourea on tumor bioenergetics and histology. Cancer Res. 1988 Feb 1;48(3):676–681. [PubMed] [Google Scholar]
- Stubbs M., Rodrigues L. M., Griffiths J. R. Potential artefacts from overlying tissues in 31P NMR spectra of subcutaneously implanted rat tumours. NMR Biomed. 1989 Apr;1(4):165–170. doi: 10.1002/nbm.1940010403. [DOI] [PubMed] [Google Scholar]
- Tozer G. M., Bhujwalla Z. M., Griffiths J. R., Maxwell R. J. Phosphorus-31 magnetic resonance spectroscopy and blood perfusion of the RIF-1 tumor following X-irradiation. Int J Radiat Oncol Biol Phys. 1989 Jan;16(1):155–164. doi: 10.1016/0360-3016(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Wehrle J. P., Li S. J., Rajan S. S., Steen R. G., Glickson J. D. 31P and 1H NMR spectroscopy of tumors in vivo: untreated growth and response to chemotherapy. Ann N Y Acad Sci. 1987;508:200–215. doi: 10.1111/j.1749-6632.1987.tb32905.x. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Gusterson B., Humphreys J., Monaghan P., Coombes R. C., Rudland P., Neville A. M. N-methyl-N-nitrosourea-induced rat mammary tumors. Hormone responsiveness but lack of spontaneous metastasis. J Natl Cancer Inst. 1981 Jan;66(1):147–155. [PubMed] [Google Scholar]





