Abstract
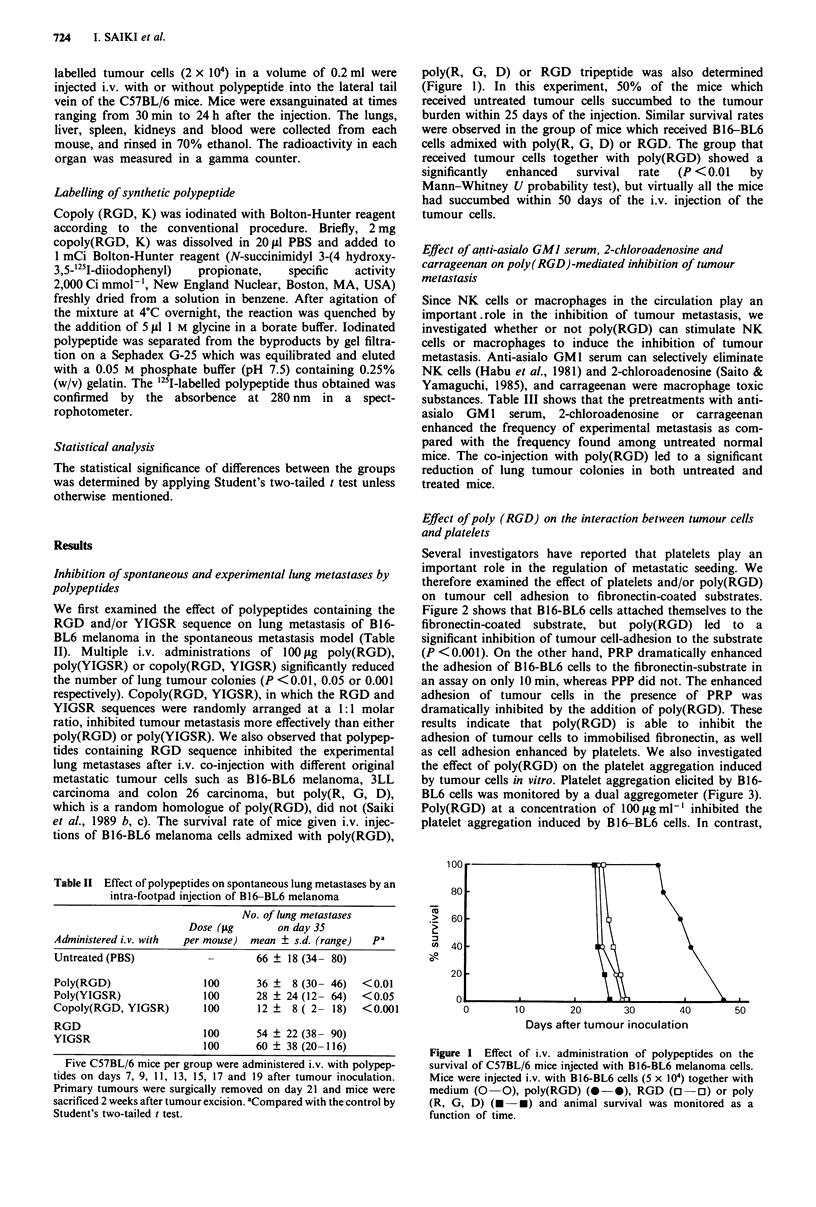
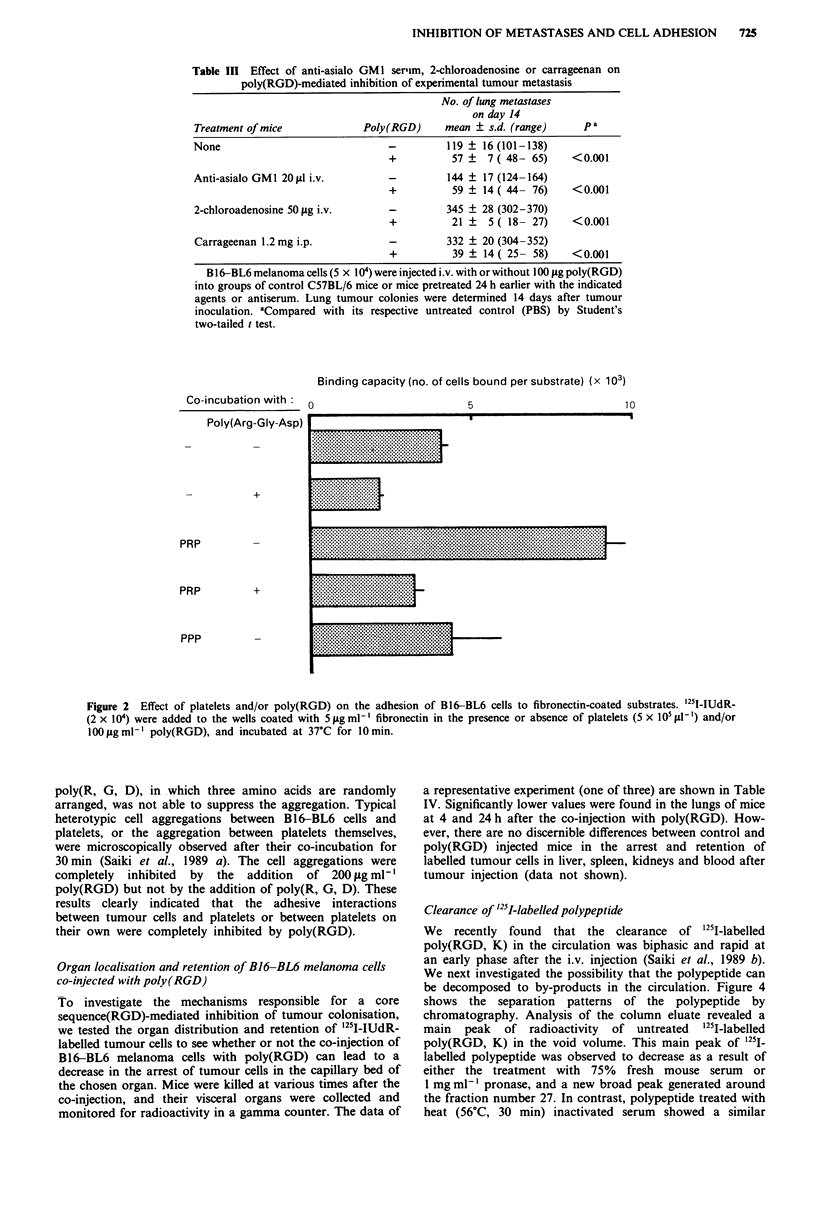
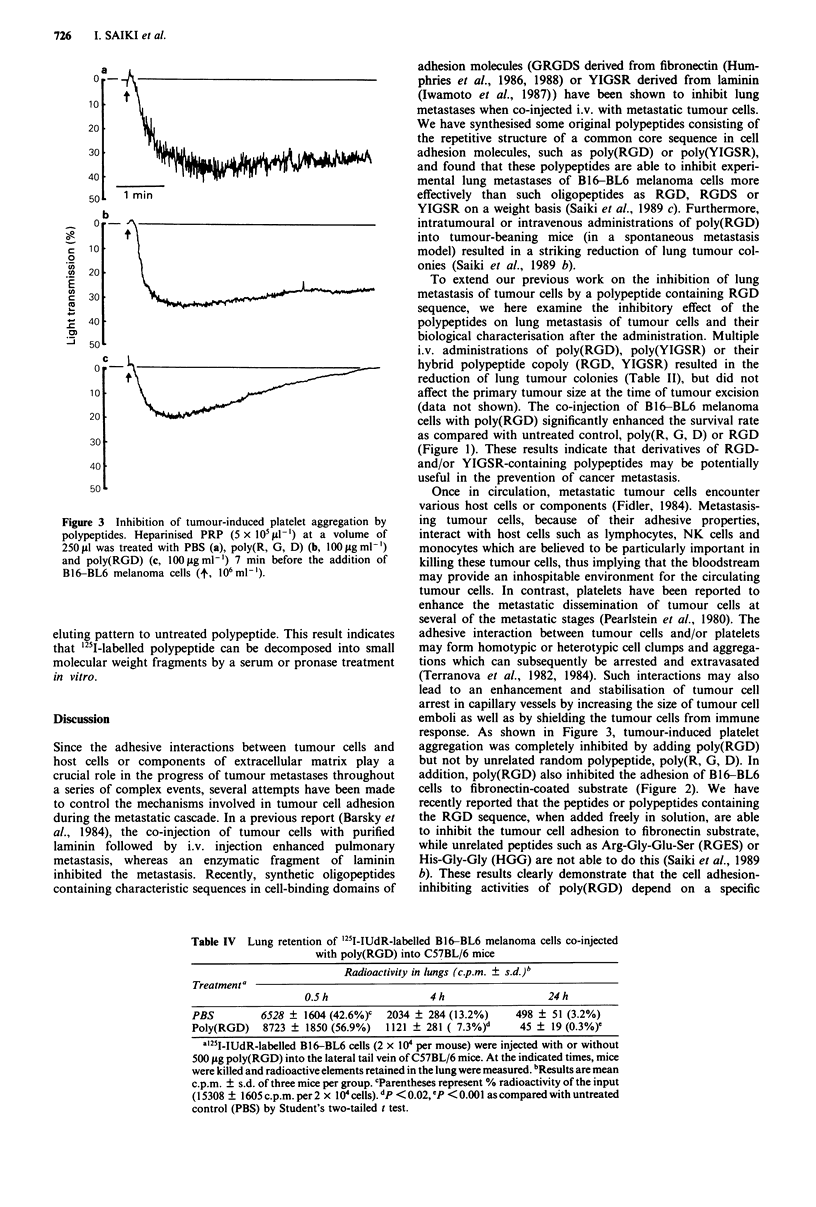
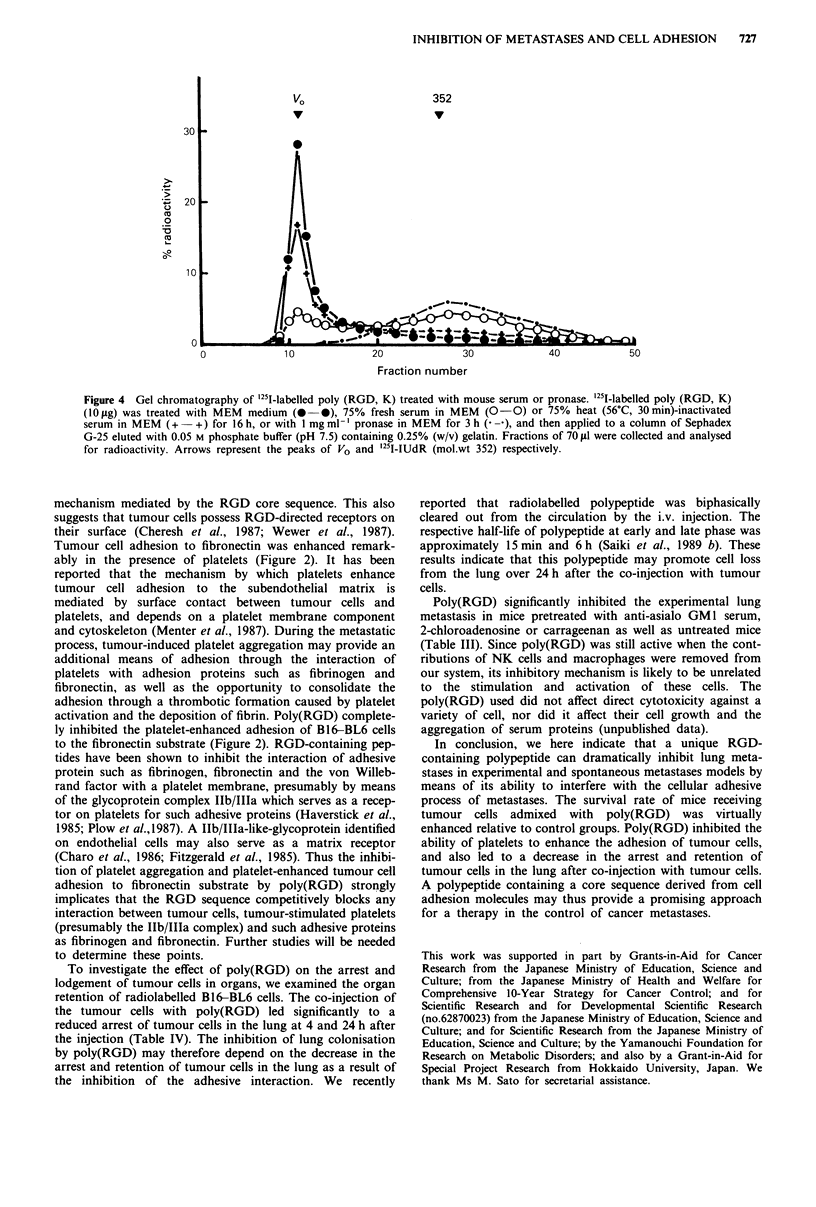
We have investigated the inhibitory effect on experimental or spontaneous lung metastases of polypeptides which contain repetitive structures of the Arg-Gly-Asp (RGD) or Tyr-Ile-Gly-Ser-Arg (YIGSR) sequence derived from adhesion molecules, and studied their biological characterisation after administration. In the spontaneous metastasis model, multiple intravenous (i.v.) administrations of poly (RGD) and poly (YIGSR) resulted in a reduction of lung tumour colonies, although the monomer peptides, RGD or YIGSR, had no effect under these conditions. The treatment with poly(RGD) substantially prolonged the survival time for mice injected i.v. with B16-BL6 cells as compared to the treatment with RGD and random poly(R, G, D). Tumour cell adhesion to the fibronectin-substrates was remarkably inhibited by adding poly(RGD) freely in solution. Poly(RGD) was found to inhibit completely the ability of platelets to enhance tumour cell adhesion to fibronectin-substrate and tumour cell-elicited platelet aggregation in vitro, but poly(R, G, D) had no such effect. We also found that poly(RGD) led to a decrease in the arrest and retention of tumour cells after its co-injection with radiolabelled tumour cells and that the radiolabelled polypeptide can be at least decomposed into small fragments during circulation. Poly(RGD) was found to be still active in inhibiting experimental lung metastasis even when the contributions of NK cells or macrophages were removed from this system after pretreatment with anti-asialo GM1 serum, 2-chloroadenosine or carrageenan. The results indicate that the poly(RGD)-mediated inhibition of tumour metastasis may be due to the interference of the adhesive interaction of tumour cells with a specific site in the target organs. Derivatives of polypeptides which contain RGD and/or YIGSR sequences derived from cell adhesion proteins may thus provide a promising approach for the control and prevention of cancer metastasis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada K. M. Synthetic peptides competitively inhibit both direct binding to fibroblasts and functional biological assays for the purified cell-binding domain of fibronectin. J Biol Chem. 1985 Sep 5;260(19):10402–10405. [PubMed] [Google Scholar]
- Barsky S. H., Rao C. N., Williams J. E., Liotta L. A. Laminin molecular domains which alter metastasis in a murine model. J Clin Invest. 1984 Sep;74(3):843–848. doi: 10.1172/JCI111501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo I. F., Fitzgerald L. A., Steiner B., Rall S. C., Jr, Bekeart L. S., Phillips D. R. Platelet glycoproteins IIb and IIIa: evidence for a family of immunologically and structurally related glycoproteins in mammalian cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8351–8355. doi: 10.1073/pnas.83.21.8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Pytela R., Pierschbacher M. D., Klier F. G., Ruoslahti E., Reisfeld R. A. An Arg-Gly-Asp-directed receptor on the surface of human melanoma cells exists in an divalent cation-dependent functional complex with the disialoganglioside GD2. J Cell Biol. 1987 Sep;105(3):1163–1173. doi: 10.1083/jcb.105.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J., Sone S., Fogler W. E., Barnes Z. L. Eradication of spontaneous metastases and activation of alveolar macrophages by intravenous injection of liposomes containing muramyl dipeptide. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1680–1684. doi: 10.1073/pnas.78.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald L. A., Charo I. F., Phillips D. R. Human and bovine endothelial cells synthesize membrane proteins similar to human platelet glycoproteins IIb and IIIa. J Biol Chem. 1985 Sep 15;260(20):10893–10896. [PubMed] [Google Scholar]
- Gasic G. J., Gasic T. B., Galanti N., Johnson T., Murphy S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int J Cancer. 1973 May;11(3):704–718. doi: 10.1002/ijc.2910110322. [DOI] [PubMed] [Google Scholar]
- Hanna N. The role of natural killer cells in the control of tumor growth and metastasis. Biochim Biophys Acta. 1985;780(3):213–226. doi: 10.1016/0304-419x(85)90004-6. [DOI] [PubMed] [Google Scholar]
- Hart I. R. The selection and characterization of an invasive variant of the B16 melanoma. Am J Pathol. 1979 Dec;97(3):587–600. [PMC free article] [PubMed] [Google Scholar]
- Haverstick D. M., Cowan J. F., Yamada K. M., Santoro S. A. Inhibition of platelet adhesion to fibronectin, fibrinogen, and von Willebrand factor substrates by a synthetic tetrapeptide derived from the cell-binding domain of fibronectin. Blood. 1985 Oct;66(4):946–952. [PubMed] [Google Scholar]
- Hayman E. G., Pierschbacher M. D., Ruoslahti E. Detachment of cells from culture substrate by soluble fibronectin peptides. J Cell Biol. 1985 Jun;100(6):1948–1954. doi: 10.1083/jcb.100.6.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Akiyama S. K., Komoriya A., Olden K., Yamada K. M. Identification of an alternatively spliced site in human plasma fibronectin that mediates cell type-specific adhesion. J Cell Biol. 1986 Dec;103(6 Pt 2):2637–2647. doi: 10.1083/jcb.103.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Olden K., Yamada K. M. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986 Jul 25;233(4762):467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Yamada K. M., Olden K. Investigation of the biological effects of anti-cell adhesive synthetic peptides that inhibit experimental metastasis of B16-F10 murine melanoma cells. J Clin Invest. 1988 Mar;81(3):782–790. doi: 10.1172/JCI113384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto Y., Robey F. A., Graf J., Sasaki M., Kleinman H. K., Yamada Y., Martin G. R. YIGSR, a synthetic laminin pentapeptide, inhibits experimental metastasis formation. Science. 1987 Nov 20;238(4830):1132–1134. doi: 10.1126/science.2961059. [DOI] [PubMed] [Google Scholar]
- Kohga S., Kinjo M., Tanaka K., Ogawa H., Ishihara M., Tanaka N. Effects of 5-(2-chlorobenzyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine hydrochloride (Ticlopidine), a platelet aggregation inhibitor, on blood-borne metastasis. Cancer Res. 1981 Nov;41(11 Pt 1):4710–4714. [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy J. B., Furcht L. T. Laminin and fibronectin promote the haptotactic migration of B16 mouse melanoma cells in vitro. J Cell Biol. 1984 Apr;98(4):1474–1480. doi: 10.1083/jcb.98.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter D. G., Sloane B. F., Steinert B. W., Onoda J., Craig R., Harkins C., Taylor J. D., Honn K. V. Platelet enhancement of tumor cell adhesion to subendothelial matrix: role of platelet cytoskeleton and platelet membrane. J Natl Cancer Inst. 1987 Nov;79(5):1077–1090. [PubMed] [Google Scholar]
- Murata J., Saiki I., Azuma I., Nishi N. Inhibitory effect of a synthetic polypeptide, poly(Tyr-Ile-Gly-Ser-Arg), on the metastatic formation of malignant tumour cells. Int J Biol Macromol. 1989 Apr;11(2):97–99. doi: 10.1016/0141-8130(89)90049-4. [DOI] [PubMed] [Google Scholar]
- Mussoni L., Poggi A., De Gaetano G., Donati M. B. Effect of ditazole, an inhibitor of platelet aggregation, on a metastasizing tumour in mice. Br J Cancer. 1978 Jan;37(1):126–129. doi: 10.1038/bjc.1978.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein E., Salk P. L., Yogeeswaran G., Karpatkin S. Correlation between spontaneous metastatic potential, platelet-aggregating activity of cell surface extracts, and cell surface sialylation in 10 metastatic-variant derivatives of a rat renal sarcoma cell line. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4336–4339. doi: 10.1073/pnas.77.7.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G., Ginsberg M. H. Arginyl-glycyl-aspartic acid sequences and fibrinogen binding to platelets. Blood. 1987 Jul;70(1):110–115. [PubMed] [Google Scholar]
- Saiki I., Iida J., Murata J., Ogawa R., Nishi N., Sugimura K., Tokura S., Azuma I. Inhibition of the metastasis of murine malignant melanoma by synthetic polymeric peptides containing core sequences of cell-adhesive molecules. Cancer Res. 1989 Jul 15;49(14):3815–3822. [PubMed] [Google Scholar]
- Saiki I., Murata J., Iida J., Nishi N., Sugimura K., Azuma I. The inhibition of murine lung metastasis by synthetic polypeptides [poly(arg-gly-asp) and poly(tyr-ile-gly-ser-arg)] with a core sequence of cell adhesion molecules. Br J Cancer. 1989 Feb;59(2):194–197. doi: 10.1038/bjc.1989.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki I., Nayar R., Bucana C., Fidler I. J. A microassay for the rapid and selective binding of cells from solid tumors to mouse macrophages. Cancer Immunol Immunother. 1986;22(2):125–131. doi: 10.1007/BF00199126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Yamaguchi J. 2-Chloroadenosine: a selective lethal effect to mouse macrophages and its mechanism. J Immunol. 1985 Mar;134(3):1815–1822. [PubMed] [Google Scholar]
- Sasaki M., Kato S., Kohno K., Martin G. R., Yamada Y. Sequence of the cDNA encoding the laminin B1 chain reveals a multidomain protein containing cysteine-rich repeats. Proc Natl Acad Sci U S A. 1987 Feb;84(4):935–939. doi: 10.1073/pnas.84.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Yamada Y. The laminin B2 chain has a multidomain structure homologous to the B1 chain. J Biol Chem. 1987 Dec 15;262(35):17111–17117. [PubMed] [Google Scholar]
- Suzuki S., Oldberg A., Hayman E. G., Pierschbacher M. D., Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J. 1985 Oct;4(10):2519–2524. doi: 10.1002/j.1460-2075.1985.tb03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova V. P., Liotta L. A., Russo R. G., Martin G. R. Role of laminin in the attachment and metastasis of murine tumor cells. Cancer Res. 1982 Jun;42(6):2265–2269. [PubMed] [Google Scholar]
- Terranova V. P., Williams J. E., Liotta L. A., Martin G. R. Modulation of the metastatic activity of melanoma cells by laminin and fibronectin. Science. 1984 Nov 23;226(4677):982–985. doi: 10.1126/science.6505678. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Dualistic nature of adhesive protein function: fibronectin and its biologically active peptide fragments can autoinhibit fibronectin function. J Cell Biol. 1984 Jul;99(1 Pt 1):29–36. doi: 10.1083/jcb.99.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Peptide inhibitors of fibronectin, laminin, and other adhesion molecules: unique and shared features. J Cell Physiol. 1987 Jan;130(1):21–28. doi: 10.1002/jcp.1041300105. [DOI] [PubMed] [Google Scholar]


