Abstract
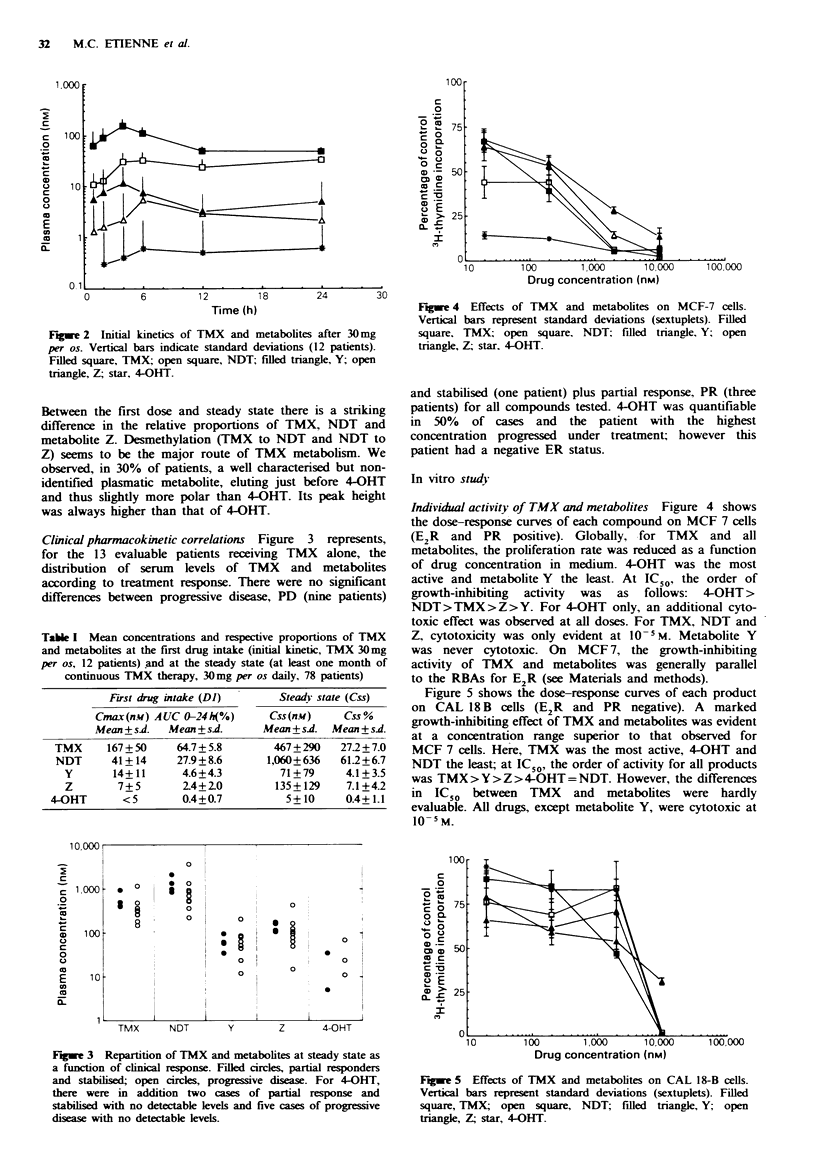
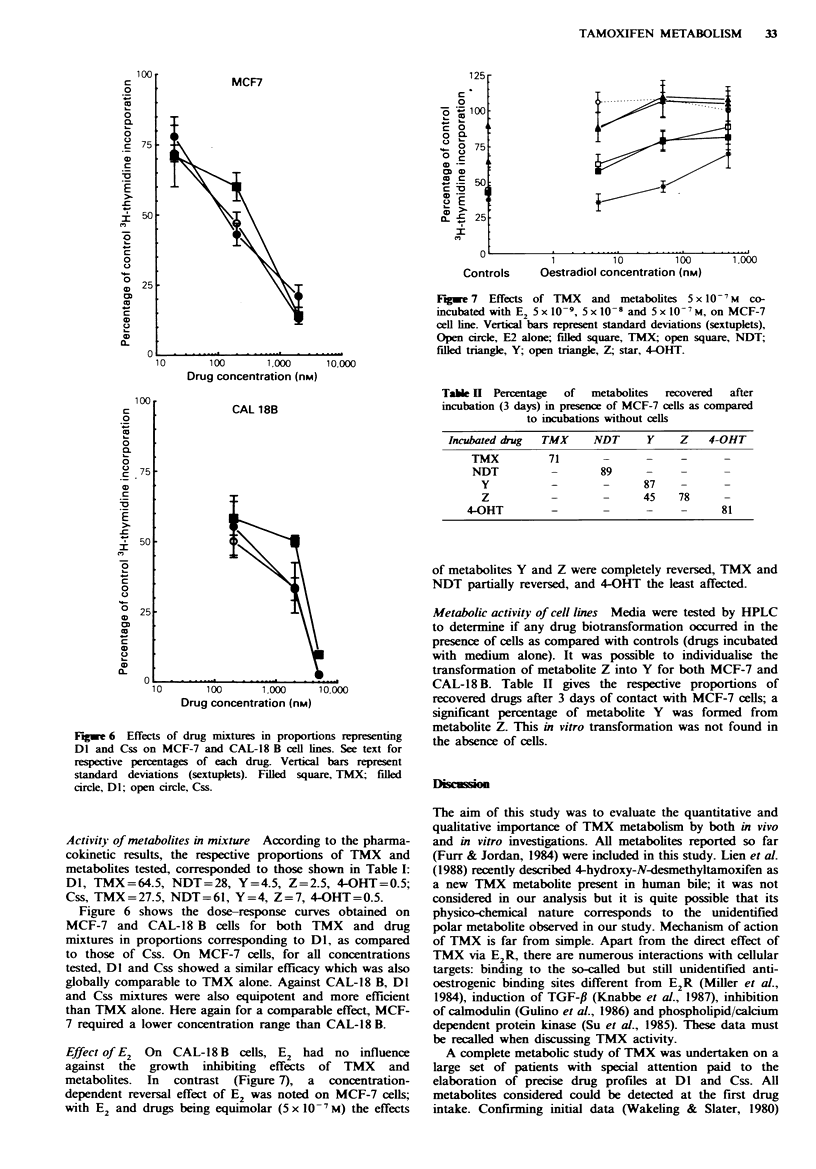
The qualitative and quantitative importance of tamoxifen (TMX) metabolism in vivo led us to investigate further the metabolic profile of this major anti-oestrogenic drug in a significant group of 81 breast cancer patients and to evaluate the respective in vitro activity of each metabolite. TMX and its four metabolites described until now (NDT, 4-OHT, Y, Z) were measured in blood (HPLC method) at the time of first drug intake and at the steady state. Between these two states, the unchanged drug relative proportion dropped from 65% to 27%. Demethylation was the major metabolic pathway. For 13 clinically evaluable patients, there was no significant difference in the distribution of serum levels of TMX and metabolites as a function of response to treatment. In vitro studies were performed on two human breast cancer cell lines: MCF-7, oestrogen receptor and progesterone receptor positive (ER+, PR+) and CAL-18 B (ER-, PR-). Cytostatic effects were evaluated by the tritiated thymidine incorporation test. TMX and all metabolites were active on these two cell lines, but the 50% inhibitory concentrations (IC50) were 4-250-fold higher in CAL-18 B than in MCF-7, depending on the metabolite considered. For the MCF-7 cells only, the antiproliferating activity was parallel to the relative binding affinity for ER. Moreover, for the MCF-7 cells only, the effects of these drugs were partially reversed by oestradiol (E2), the higher the metabolite affinity for ER, the lower the reversal efficacy. These compounds were tested in mixtures at proportions duplicating those found in patients after initial drug intake (mixture D1), and the steady state (mixture Css). The mixtures were also compared to the equimolar unchanged drug. No differences were seen among these three experimental conditions for either MCF-7 or CAL-18 B. A dose-effect relationship was noted. Overall, TMX and its metabolites exert a dual effect: when concentrations are below a threshold between 2 x 10(-6) and 10(-5) M, the drugs are mainly cytostatic; this effect is related to their affinity for ER. At higher relevant clinical concentrations, a cytotoxic activity is observed and it appears independent of the presence of ER.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam H. K., Patterson J. S., Kemp J. V. Studies on the metabolism and pharmacokinetics of tamoxifen in normal volunteers. Cancer Treat Rep. 1980 Jun-Jul;64(6-7):761–764. [PubMed] [Google Scholar]
- Briand P., Lykkesfeldt A. E. Effect of estrogen and antiestrogen on the human breast cancer cell line MCF-7 adapted to growth at low serum concentration. Cancer Res. 1984 Mar;44(3):1114–1119. [PubMed] [Google Scholar]
- Fisher B., Brown A., Wolmark N., Redmond C., Wickerham D. L., Wittliff J., Dimitrov N., Legault-Poisson S., Schipper H., Prager D. Prolonging tamoxifen therapy for primary breast cancer. Findings from the National Surgical Adjuvant Breast and Bowel Project clinical trial. Ann Intern Med. 1987 May;106(5):649–654. doi: 10.7326/0003-4819-106-5-649. [DOI] [PubMed] [Google Scholar]
- Furr B. J., Jordan V. C. The pharmacology and clinical uses of tamoxifen. Pharmacol Ther. 1984;25(2):127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- Gioanni J., Courdi A., Lalanne C. M., Fischel J. L., Zanghellini E., Lambert J. C., Ettore F., Namer M. Establishment, characterization, chemosensitivity, and radiosensitivity of two different cell lines derived from a human breast cancer biopsy. Cancer Res. 1985 Mar;45(3):1246–1258. [PubMed] [Google Scholar]
- Gulino A., Barrera G., Vacca A., Farina A., Ferretti C., Screpanti I., Dianzani M. U., Frati L. Calmodulin antagonism and growth-inhibiting activity of triphenylethylene antiestrogens in MCF-7 human breast cancer cells. Cancer Res. 1986 Dec;46(12 Pt 1):6274–6278. [PubMed] [Google Scholar]
- Hayward J. L., Rubens R. D., Carbone P. P., Heuson J. C., Kumaoka S., Segaloff A. Assessment of response to therapy in advanced breast cancer. A project of the programme on clinical oncology of the International Union against Cancer, Geneva, Switzerland. Eur J Cancer. 1978 Nov;14(11):1291–1292. doi: 10.1016/0014-2964(78)90238-4. [DOI] [PubMed] [Google Scholar]
- Jordan V. C., Bain R. R., Brown R. R., Gosden B., Santos M. A. Determination and pharmacology of a new hydroxylated metabolite of tamoxifen observed in patient sera during therapy for advanced breast cancer. Cancer Res. 1983 Mar;43(3):1446–1450. [PubMed] [Google Scholar]
- Jordan V. C. Biochemical pharmacology of antiestrogen action. Pharmacol Rev. 1984 Dec;36(4):245–276. [PubMed] [Google Scholar]
- Jordan V. C., Fritz N. F., Tormey D. C. Endocrine effects of adjuvant chemotherapy and long-term tamoxifen administration on node-positive patients with breast cancer. Cancer Res. 1987 Jan 15;47(2):624–630. [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Miller M. A., Mullick A., Sheen Y. Y. Antiestrogen action in breast cancer cells: modulation of proliferation and protein synthesis, and interaction with estrogen receptors and additional antiestrogen binding sites. Breast Cancer Res Treat. 1985;5(3):231–243. doi: 10.1007/BF01806018. [DOI] [PubMed] [Google Scholar]
- Kemp J. V., Adam H. K., Wakeling A. E., Slater R. Identification and biological activity of tamoxifen metabolites in human serum. Biochem Pharmacol. 1983 Jul 1;32(13):2045–2052. doi: 10.1016/0006-2952(83)90425-2. [DOI] [PubMed] [Google Scholar]
- Knabbe C., Lippman M. E., Wakefield L. M., Flanders K. C., Kasid A., Derynck R., Dickson R. B. Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell. 1987 Feb 13;48(3):417–428. doi: 10.1016/0092-8674(87)90193-0. [DOI] [PubMed] [Google Scholar]
- Lien E. A., Solheim E., Kvinnsland S., Ueland P. M. Identification of 4-hydroxy-N-desmethyltamoxifen as a metabolite of tamoxifen in human bile. Cancer Res. 1988 Apr 15;48(8):2304–2308. [PubMed] [Google Scholar]
- Löser R., Seibel K., Roos W., Eppenberger U. In vivo and in vitro antiestrogenic action of 3-hydroxytamoxifen, tamoxifen and 4-hydroxytamoxifen. Eur J Cancer Clin Oncol. 1985 Aug;21(8):985–990. doi: 10.1016/0277-5379(85)90119-1. [DOI] [PubMed] [Google Scholar]
- Manni A., Arafah B. M. Tamoxifen-induced remission in breast cancer by escalating the dose to 40 mg daily after progression on 20 mg daily: a case report and review of the literature. Cancer. 1981 Aug 15;48(4):873–875. doi: 10.1002/1097-0142(19810815)48:4<873::aid-cncr2820480402>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Milano G., Etienne M. C., Frenay M., Khater R., Formento J. L., Renee N., Moll J. L., Francoual M., Berto M., Namer M. Optimised analysis of tamoxifen and its main metabolites in the plasma and cytosol of mammary tumours. Br J Cancer. 1987 May;55(5):509–512. doi: 10.1038/bjc.1987.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milano G., Moll J. L., Formento J. L., Francoual M., Krebs B. P., Namer M., Boublil J. L., Lalanne C. M. Simultaneous micro measurement of steroid receptors in breast cancer. Br J Cancer. 1983 Oct;48(4):579–584. doi: 10.1038/bjc.1983.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A., Lippman M. E., Katzenellenbogen B. S. Antiestrogen binding in antiestrogen growth-resistant estrogen-responsive clonal variants of MCF-7 human breast cancer cells. Cancer Res. 1984 Nov;44(11):5038–5045. [PubMed] [Google Scholar]
- Reddel R. R., Murphy L. C., Hall R. E., Sutherland R. L. Differential sensitivity of human breast cancer cell lines to the growth-inhibitory effects of tamoxifen. Cancer Res. 1985 Apr;45(4):1525–1531. [PubMed] [Google Scholar]
- Reddel R. R., Murphy L. C., Sutherland R. L. Effects of biologically active metabolites of tamoxifen on the proliferation kinetics of MCF-7 human breast cancer cells in vitro. Cancer Res. 1983 Oct;43(10):4618–4624. [PubMed] [Google Scholar]
- Shek L. L., Godolphin W., Spinelli J. J. Oestrogen receptors, nodes and stage as predictors of post-recurrence survival in 457 breast cancer patients. Br J Cancer. 1987 Dec;56(6):825–829. doi: 10.1038/bjc.1987.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J. F., Minton M. J., Rubens R. D. Trial of tamoxifen at a dose of 40 mg daily after disease progression during tamoxifen therapy at a dose of 20 mg daily. Cancer Treat Rep. 1982 Jun;66(6):1445–1446. [PubMed] [Google Scholar]
- Su H. D., Mazzei G. J., Vogler W. R., Kuo J. F. Effect of tamoxifen, a nonsteroidal antiestrogen, on phospholipid/calcium-dependent protein kinase and phosphorylation of its endogenous substrate proteins from the rat brain and ovary. Biochem Pharmacol. 1985 Oct 15;34(20):3649–3653. doi: 10.1016/0006-2952(85)90225-4. [DOI] [PubMed] [Google Scholar]
- Taylor C. M., Blanchard B., Zava D. T. Estrogen receptor-mediated and cytotoxic effects of the antiestrogens tamoxifen and 4-hydroxytamoxifen. Cancer Res. 1984 Apr;44(4):1409–1414. [PubMed] [Google Scholar]
- Vogel C. L., East D. R., Voigt W., Thomsen S. Response to tamoxifen in estrogen receptor-poor metastatic breast cancer. Cancer. 1987 Sep 15;60(6):1184–1189. doi: 10.1002/1097-0142(19870915)60:6<1184::aid-cncr2820600605>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Wakeling A. E., Slater S. R. Estrogen-receptor binding and biologic activity of tamoxifen and its metabolites. Cancer Treat Rep. 1980 Jun-Jul;64(6-7):741–744. [PubMed] [Google Scholar]
- Watkins S. M. The value of high dose tamoxifen in postmenopausal breast cancer patients progressing on standard doses: a pilot study. Br J Cancer. 1988 Mar;57(3):320–321. doi: 10.1038/bjc.1988.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson P. M., Ribiero G. G., Adam H. K., Kemp J. V., Patterson J. S. Tamoxifen (Nolvadex) therapy--radionale for loading dose followed by maintenance dose for patients with metastatic breast cancer. Cancer Chemother Pharmacol. 1982 Dec;10(1):33–35. doi: 10.1007/BF00257234. [DOI] [PubMed] [Google Scholar]


