Abstract
Background
Previous studies suggest that markers of inflammation are elevated in patients with atrial fibrillation (AF). However, because inflammation has been implicated in contributing to risk of both AF and coronary artery disease (CAD), which are often present in the same populations, it is important to control for confounding by the presence of CAD. We therefore examined several biomarkers of inflammation and ultimately genotyped IL-6 polymorphisms in AF patients in a cohort of subjects with known CAD.
Methods
We performed a cross-sectional analysis of 971 participants in the Heart and Soul Study, 46 of whom had AF. IL-6, CRP, tumor necrosis factor-α, CD-40 ligand, monocyte chemoattractant protein-1, and fibrinogen levels were measured.
Results
In both unadjusted and adjusted analyses, IL-06 was the only biomarker significantly associated with AF (median IL-6 3.76 pg/ml and 2.52 pg/ml in those with and without AF, respectively, p=0.0005; adjusted odds ratio [OR] 1.77 p=0.032). The IL-6 –174CC genotype was significantly associated with the presence of AF in the adjusted analysis (OR 2.34, p=0.04) and with higher IL-6 levels (p=0.002).
Conclusions
In this cohort of subjects with CAD, AF was significantly associated with elevated IL-6 levels and the IL-6 –174CC genotype. No associations were found with other biomarkers, including CRP. This suggests that IL-6 is a uniquely important mediator in the pathophysiology of AF.
Atrial fibrillation (AF) is the most common arrhythmia and is responsible for substantial morbidity and mortality.1 While the precise etiology remains unknown, animal2,3 and human4,5 studies have suggested that inflammation and fibrosis play an important role in some types of AF. Earlier studies found C-reactive protein (CRP) to be elevated in patients with AF.6 However, more recent investigation has suggested that the elevation in CRP may simply reflect underlying disease processes rather than something unique to AF itself.7 In particular, coronary artery disease is an important potential confounder because it is significantly associated with both elevated CRP levels8 and a greater risk of AF.9 However, to our knowledge, no previous study has determined if the relationship between inflammatory markers and AF persists in a population restricted to subjects with known coronary artery disease.
In fact, the mechanism by which inflammation and stimulation of CRP secretion occurs in AF remains unknown, and more recent studies have suggested that one of the most important stimuli of CRP release,10 interleukin 6 (IL-6), may play an important role.11,12 IL-6 has been shown to significantly correlate with increased left atrial size,13 an important risk factor for developing AF. In addition, polymorphisms in the promoter region of the IL-6 gene have been associated with post-operative AF.14,15 Importantly, IL-6 has not previously been shown to be independently associated with spontaneous (non-post-operative) AF nor have polymorphisms of the IL-6 gene been studied in these patients.
We examined the relationship between several serologic markers of inflammation and the presence of AF in a well-characterized cohort of patients, all of whom had coronary artery disease.
Methods
Patients
We performed an analysis of baseline electrocardiograms (ECGs) and serum inflammatory markers collected for the Heart and Soul Study. The Heart and Soul Study is a prospective cohort study of health outcomes in patients with coronary artery disease. The details of eligibility and enrollment have been described previously.16 Briefly, between September 2000 and December 2002, 1024 outpatients with stable coronary artery disease were recruited using administrative databases from two Department of Veterans Affairs Medical Centers (San Francisco, California and Palo Alto, California), one university medical center (University of California, San Francisco), and nine public health clinics in the Community Health Network of San Francisco. Patients were eligible to participate if they had a history of myocardial infarction (MI), angiographic evidence of >= 50% stenosis in one or more coronary vessels, evidence of exercise-induced ischemia by treadmill or nuclear testing, or prior coronary revascularization. Patients were not eligible if they had an acute coronary syndrome within the past six months, could not walk one block, or were planning to move out of the local area within three years.
In all participants, the cardiac rhythm was determined from a baseline 12-lead ECG at the time of enrollment. We utilized this data to perform a cross-sectional study. Patients with atrial flutter (n=3), a multifocal atrial rhythm (n=1), ventricular arrhythmia (n=0), or a regular supraventricular tachycardia (n=1) on their baseline ECG were excluded. An additional 32 patients were unable to provide blood samples (2 with AF), leaving a total of 971 participants for the analysis.
The institutional review board at each of the sites approved this protocol. All participants provided written informed consent.
Measurements
Atrial Fibrillation
On the day of their visit, all patients underwent a standard 12 lead electrocardiogram (ECG) with standard amplitudes obtained at a 25 mm/s paper speed. The diagnosis of AF was adjudicated by 2 physicians, and discrepancies resolved by a 3rd physician reviewer. Only patients whose rhythm was atrial fibrillation, sinus rhythm or an atrial paced rhythm were included in the analysis.
Inflammatory Biomarkers
Participants were instructed to fast for 12 hours (except for medication, which they were able to take with water), not to take aspirin for one week, and not to smoke for five hours prior to their study appointment. Venous blood samples were obtained, and plasma and serum samples were stored at −70 degrees Celsius. Laboratory technicians who assayed the inflammatory markers were blinded to clinical characteristics.
Serum fibrinogen concentrations were determined by the Clauss assay. The Roche (Indianapolis, Indiana) Integra high-sensitivity assay was used to measure CRP in the first 229 participants and (due to a change in the laboratory) the Beckman (Galway, Ireland) Extended Range assay to measure CRP in the remaining samples. Results from these two assays were highly correlated (r = 0.99 in 185 participants). The R&D Systems (Minneapolis, MN) Quantikine HS IL-6 Immunoassay was used to determine the concentration of IL-6. The R&D Systems Quantikine HS CD40 Ligand Immunoassay was used to measure serum CD40 ligand using the quantitative sandwich enzyme immunoassay technique (lower limit of detection. The Human Serum Adipokine Panel B LINCOplex Kit (Linco Research, Inc., St. Charles, MO) was used to measure tumor necrosis factor-α (TNF-α) and monocyte cheomoattractant protein-1 (MCP-1).
Single Nucleotide Polymorphisms
Whole blood was collected for genetic analysis from 986 participants, and buffy coat samples were stored in a freezer at −80 degrees Celsius. Genomic DNA was extracted from peripheral blood lymphocytes using a salt modification method (Gentra Systems). The IL6-174G/C (rs1800795) and IL6-572G/C (rs1800796) polymorphisms (SNPs) were genotyped by template-directed dye-terminator incorporation assay with fluorescence polarization detection,17 using the AcycloPrime-FP kit (Perkin-Elmer) as previously described.18 Genotyping was performed by investigators blinded to clinical status. Quality control included negative (blank) and positive controls on each 96-sample plate (44 duplicates, 100% concordance observed). Genotyping assays were completed in 871 of the 986 samples.
Other Measurements
Age, medical history, and smoking status were determined by questionnaire. We measured height and weight and calculated body mass index (kg/m2). Participants were instructed to bring their medication bottles to the study appointment and all medications were recorded. A complete resting echocardiogram was performed using an Acuson Sequoia Ultrasound System with a 3.5 MHz transducer with standard measurements as previously described.16 Results from all echocardiograms were interpreted by an expert echocardiographer blinded to the results of the serologic markers and genetic analyses.
Statistical Analysis
Normally distributed continuous variables are presented as means ± SD. Continuous variables that were not normally distributed are presented as medians and interquartile ranges (IQR). Consistent with previous studies, IL-613,19 and CRP6 were right-skewed. TNF-α was noted to be significantly right-skewed as well. Baseline characteristics were assessed using t-tests and Spearman’s rank correlation coefficient for continuous variables and χ2 tests for categorical variables. The bivariate associations between each serologic marker and atrial fibrillation were performed using t-tests and the Wilcoxon rank sum test as appropriate.
IL-6, CRP, TNF-α were each log-transformed for the multiple logistic regression model in order to improve linear fitting. Multiple logistic regression analysis was performed for each marker as a continuous variable and after categorizing each marker into quartiles. Covariates that were clinically known to be important to atrial fibrillation and the presence of inflammation (including age, gender, race, hypertension, ejection fraction) 1,20 as well as variables significantly associated with the predictors (inflammatory markers) and AF in the present cohort were included in the model. Potentially important intermediaries were added to the model to evaluate mediation.
The SNPs were analyzed for dominant and recessive effects by recoding each polymorphism as a dichotomous variable (major allele homozygous versus other; minor alelle homozygous versus other) and in an additive model (by allele frequency). χ2 tests were used to assess the relationships between each SNP and AF. Multiple logistic regression was performed using the dichotomous SNP variables and including the same covariates in the model included as above. Differences between IL-6 levels for each genotype across each SNP were assessed using the Kruskal–Wallis rank test.
Results
Of the 971 participants, 46 (5%) had AF and 925 (95%) had sinus rhythm (patients with an atrial paced rhythm [n=11] were included in the sinus rhythm group). The baseline demographics and clinical characteristics of these patients are shown in Table I.
Table I.
Baseline characteristics of subjects with and without atrial fibrillation on their baseline electrocardiogram.
| AF (n=46) | No AF (n=925) | P value | |
|---|---|---|---|
| Age (years) | 74 ± 10 | 66 ± 11 | <0.0001 |
| Male | 43 (94%) | 750 (81%) | 0.034 |
|
White
Black Asian Hispanic |
40 (87%)
0 4 (9%) 1 (2%) |
548 (59%)
155 (17%) 107 (12%) 84 (9%) |
0.003; pairwise differences significant for whites (p<0.001) and blacks (0.002) |
| Hypertension | 27 (60%) | 654 (71%) | 0.12 |
| Diabetes | 8 (18%) | 248 (27%) | 0.20 |
| Prior myocardial infarction | 22 (50%) | 496 (54%) | 0.61 |
| Heart failure | 12 (26%) | 206 (22%) | 0.55 |
| Ejection fraction (%) | 60 ± 9 | 62 ± 10 | 0.17 |
| Left ventricular mass index (g/m2) | 100 ± 24 | 100 ± 34 | 0.96 |
| Left atrial volume (ml) | 101 ± 36 | 62 ± 21 | <0.0001 |
| Body mass index (kg/m2) | 30 ± 8 | 28 ± 5 | 0.10 |
| Statin use | 25 (54%) | 604 (65%) | 0.13 |
| Ace inhibitor or angiotensin receptor blocker use | 29 (63%) | 467 (51%) | 0.096 |
| Smoking | 4 (9%) | 189 (21%) | 0.057 |
| Alcohol use | 17 (38%) | 268 (29%) | 0.22 |
Inflammatory Biomarkers
IL-6 was significantly higher in subjects with AF, while CRP was not (Table II). Although a higher CD-40 ligand levels in AF patients approached statistical significance, neither this nor any of the other markers were significantly associated with AF. In a multiple logistic regression model in which each marker was included, IL-6 was the only one independently associated with AF (Figure 1).
Table II.
Levels of biomarkers in those with and without atrial fibrillation on their baseline electrocardiogram. Interquartile ranges are shown in parentheses for the biomarkers that did not exhibit a normal distribution.
| AF (n=46) | No AF (n=925) | P value | |
|---|---|---|---|
| IL-6 (pg/mL) | 3.76 (2.61–5.21) | 2.52 (1.58–4.06) | 0.0005 |
| CRP (mg/dL) | 2.46 (1.38–4.45) | 2.15 (0.89–4.9) | 0.37 |
| TNF-α (pg/mL) | 4.48 (3.22–6.95) | 3.76 (2.56–5.53) | 0.12 |
| MCP-1 (pg/mL) | 260 ± 129 | 243 ± 128 | 0.38 |
| CD-40 ligand (pg/mL) | 5910 ± 3085 | 5078 ± 3069 | 0.078 |
| Fibrinogen (mg/dL) | 392 ± 91 | 408 ± 72 | 0.23 |
Figure 1.
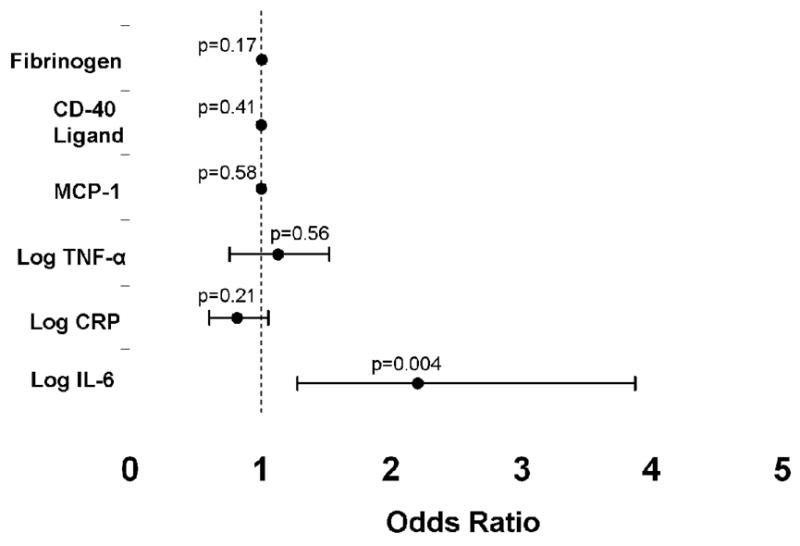
Odds ratios for the association between each biomarker and the presence of atrial fibrillation, adjusted for the independent effects of each biomarker (after entering each biomarker into a logistic regression model with atrial fibrillation as the binary outcome). Error bars denote 95% confidence intervals. The dashed line denotes an odds ratio of 1.
Each progressive quartile of IL-6 compared to the first quartile of IL-6 was associated with progressively larger odds of AF (test for trend p=0.0017, Figure 2). This association was maintained after adjusting for age, gender, hypertension, diabetes, heart failure, ejection fraction, left ventricular mass index, and smoking (test for trend p=0.033). No other markers demonstrated a significant association between progressive quartiles and larger odds of AF.
Figure 2.
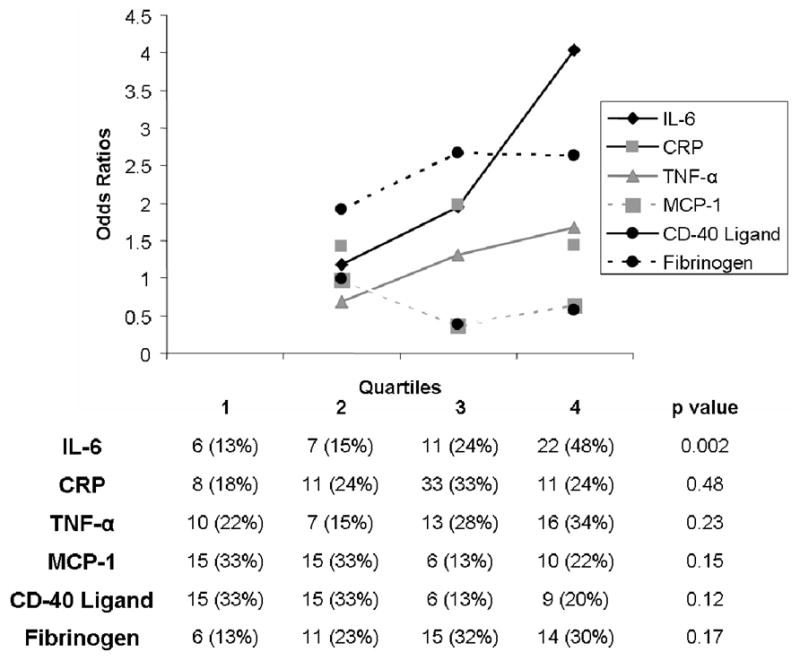
Odds ratios of the 2nd, 3rd, and 4rth quartiles compared to the first quartile for each biomarker as predictors of atrial fibrillation. The table exhibits the number of patients with atrial fibrillation in each quartile for each marker.
Table III lists continuous variables significantly correlated with IL-6. In assessing IL-6 versus categorical clinical characteristics, heart failure (p<0.0001) and smoking (p=0.0002) were significantly associated with elevated levels; no associations were found between IL=6 levels and gender, diabetes, hypertension, statin use, or ACE inhibitor/angiotensin receptor blocker use. Those with a previous myocardial infarction had higher IL-6 levels (median 2.75 pg/ml versus 2.42 pg/ml) that did not quite meet statistical significance (p=0.05). Asians had lower IL-6 levels (median 1.88 pg/ml versus median 2.68 pg/ml in the remainder of the cohort, p<0.0001), but IL-6 levels did not otherwise differ by race.
Table III.
Correlations between continuous covariates and IL-6 levels. Note that a rho≥ 0.7 generally represents a “strong” association
| Spearman’s rho(r) | P value | |
|---|---|---|
| Age | 0.15 | <0.0001 |
| Atrial volume | 0.14 | <0.0001 |
| LV mass index | 0.16 | <0.0001 |
| Ejection fraction | −0.14 | <0.0001 |
| Body mass index | 0.18 | <0.0001 |
The association between IL-6 and AF persisted in a logistic regression model controlling for age, gender, hypertension, diabetes, heart failure, ejection fraction, left ventricular mass index, and smoking (odds ratio [OR] 1.77, 95% confidence interval [CI] 1.75-2.93, p=0.025). Given the log transformation, this means that a doubling of IL-6 levels was associated with a 1.49 greater odds of AF (95% CI 1.05-2.12). Inclusion of left atrial volume attenuated the association between IL-6 and AF (OR 1.55, 95% CI 0.89–2.70, p=0.12), consistent with an intermediary effect.
Genotypes of the IL-6 polymorphisms
Eight hundred and seventy one subjects (40 with AF) were successfully genotyped for the IL-6 -174G/C and the IL-6 -572G/C SNPs. For the -174G/C SNP, 451 (52%), 302 (35%), and 123 (14%) carried the GG, GC, and CC genotypes, respectively. For the -572G/C SNP, 687 (76%), 153 (17%), and 63 (7%) carried the GG, GC, and CC genotypes, respectively. Restricting to Caucasians only, the distributions of each SNP did not deviate from Hardy-Weinberg expectations (p=0.06 for -174G/C and p=0.81 for -572G/C). However, the number of outcomes (n=34) was small in this restricted sample, and no significant associations were observed related to either of the SNPs and AF in this subgroup. Therefore, the genotype analysis includes the cohort as a whole; as expected given the multi-racial nature of the cohort, the genotype distribution of each SNP in the entire cohort deviated from Hardy-Weinberg expectations (p<0.001).
The genotype and allele frequencies for the two SNPs in patients with and without AF (with p values from Chi square analyses) are shown in Figure 3. Although not statistically significant in the unadjusted analysis, there was a trend towards a progressively greater proportion with AF from the -174GG to -174GC to -174CC genotypes (test for trend p=0.08). None of the unadjusted chi square analyses approached statistical significance in examining the relationship between AF and the - 572G/C SNP (test for trend p=0.53).
Figure 3.
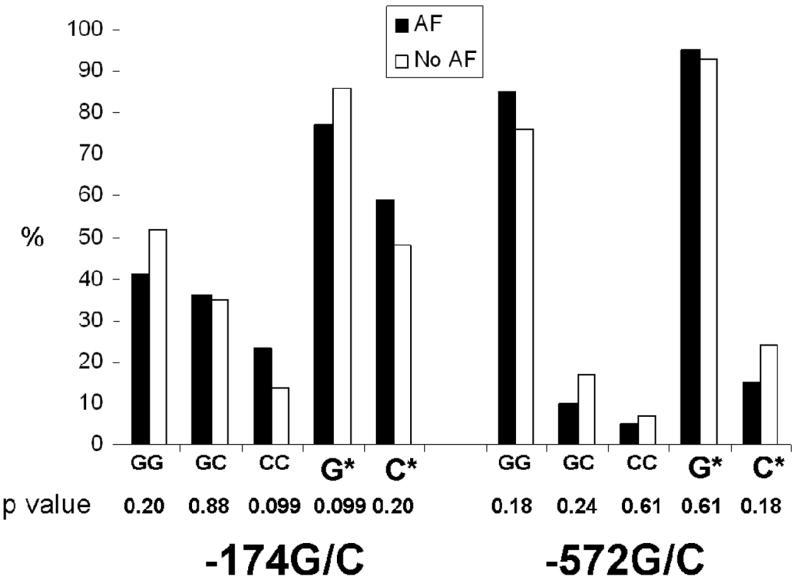
Proportions of patients with and without atrial fibrillation for each genotype and each allele (* denotes inclusion of both genotypes for a given allele). P values represent results of pairwise comparisons after collapsing for genotype or allele.
After applying the same multiple logistic regression model used in the biomarker analysis (adjusting for age, gender, hypertension, diabetes, heart failure, ejection fraction, left ventricular mass index, and smoking), the -174CC genotype was significantly more common in patients with AF (p=0.040, Figure 4).
Figure 4.

Odds ratios for the association between each -174G/C genotype and allele (*denotes inclusion of both genotypes for a given allele) and atrial fibrillation after adjustment for age, gender, hypertension, diabetes, heart failure, ejection fraction, left ventricular mass index, and smoking. Error bars denote 95% confidence intervals. The dashed line denotes an odds ratio of 1.
IL-6 polymorphisms and IL-6 levels
Genotype of both IL6 SNPs was significantly associated with serum IL6 levels. For the IL6 -174G/C SNP, serum IL-6 levels progressively increased with the number of C alleles present (highest in minor allele homozygotes, -174CC, Figure 5). For the - 572G/C polymorphism, IL-6 levels progressively decreased with the number of C alleles present (lowest in minor allele homozygotes, -572CC, Figure 5).
Figure 5.
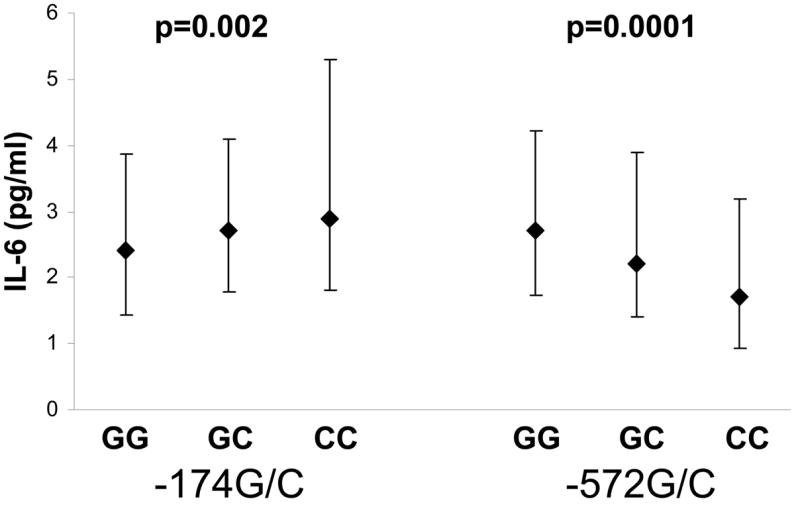
Median levels of IL-6 (pg/ml) for each genotype of the -174G/C and -572G/C polymorphisms. Error bars denote interquartile ranges.
Discussion
In a large cross-sectional cohort of patients with coronary artery disease, we found that, among 6 serologic markers of inflammation, IL-6 had a unique and independent association with AF that persisted after adjustment for multiple potential confounders. In addition, the CC genotype of the IL-6 -174G/C IL-6 polymorphism exhibited a 2.35 greater odds of being associated with AF than the GC and GG genotypes (p=0.040) in multiple logistic regression, and the same genotype (CC) also demonstrated a significant association with elevated IL-6 levels.
Although AF is the most common arrhythmia and is responsible for substantial morbidity and mortality,1 the cause remains unknown. Risk factors such as age, gender, heart failure, and coronary artery disease, are well established.9 Biopsy data suggests that AF may have an inflammatory etiology,4 but the specific mechanism remains unknown. In particular, it is unclear whether inflammation is a cause or an effect of AF and, in either case, what mechanistic pathways may be important. Chung et al. demonstrated that CRP was significantly elevated in AF.6 However, subsequent study suggests that this association may have been confounded by concomitant conditions.7 As both AF and CRP are associated with coronary artery disease, we chose to study biomarkers of inflammation in a population restricted to subjects with coronary artery disease. In this population, we were unable to find an association between AF and CRP.
We also studied several other markers previously reported to be associated with myocardial inflammation (eg, myocarditis) and/or coronary artery disease: MCP-1,21–23 TNF-α,24–26 and CD-40 ligand.27 Fibrinogen was included because of the suggestion that inflammation in AF may be related to the known aberrations in the coagulation cascade in AF.12,28 None of these markers was independently associated with AF.
We did not find a significant association between AF and CRP, potentially reflecting confounding due to underlying coronary disease in previous studies,7 bias due to choice of controls in previous case control (rather than cohort) studies, or insufficient power to detect a difference in CRP levels in this population.
To our knowledge, this is the first large cohort study in which the relationship between multiple biomarkers of inflammation and AF and the association between IL-6 and AF has been studied. IL-6 has been examined in case control studies of AF, but this study design is more likely to introduce bias. In those studies, elevated levels were consistently observed only in univariate analyses, with loss of a significant association after controlling for covariates.11,28 Of importance, IL-6 has been associated with increased left atrial size.13 Because increasing left atrial size is known to be a risk factor for AF,21 one possibility is that elevated IL-6 results in AF via left atrial remodeling. In fact, we observed an association between IL-6 and increasing left atrial volume (albeit a weak one), and, as would be expected if left atrial volume were an important intermediary, the association between IL-6 and AF was attenuated when left atrial volume was added to the logistic regression model. The mechanism by which IL-6 might result in atrial remodeling is unknown; however, animal data suggests that IL-6 may stimulate matrix metalloproteinase-2,29 a molecule previously implicated in the atrial remodeling observed in AF.30
The -174G/C and the -572G/C SNPs both lie in the promoter region of IL-6 and have been reported to modulate IL-6 expression in vitro.31–32 Although not statistically significant, there was a trend in the unadjusted analysis for IL-6 -174G/C genotypes associated with higher IL-6 levels to be found at greater frequency in the AF group. After controlling for important covariates, the -174CC genotype was significantly associated with AF. Importantly, this same genotype was associated with higher IL-6 levels.
The only other studies to examine the -174G/C SNP in AF patients involved post-operative AF after coronary artery bypass grafting, and these 2 studies, involving only 11014 and 9615 patients, reported contradictory results: although both reported that increased IL-6 levels were associated with post-operative AF, one found that the area under the curve of IL-6 over several days was higher for the GG allele14 and the other found higher acute IL-6 levels associated with the CC allele.15 The study that had results contradictory to ours was based on 24 outcomes.14 Regardless, post-operative AF is felt to be a different entity than spontaneously occurring AF,20 and ours is the first study to examine this SNP in subjects with the more common spontaneous form.
This study has several limitations. Most importantly, we erred on the side of specificity in identifying our outcome (AF). Because we did not have reliable and uniform data regarding a history of AF and because historical reports can be inaccurate, we limited our outcome to the presence of AF documented on the baseline electrocardiogram performed at the time of enrollment in this cross-sectional analysis and required that 2 blinded physicians confirm the diagnosis. As any misclassification would therefore affect only sensitivity and is almost certainly non-differential with regards to the predictors, this lack of sensitivity will bias our findings towards the null hypothesis. In other words, if anything, a reduced sensitivity in this case would increase the probability of a type II error, that we would miss a difference when one actually existed; because of the high specificity (which should be at or near 100%), it is crucial to emphasize that significant differences in outcome groups that were detected (such as those seen with IL-6 levels and the polymorphisms) are almost certainly real (the design works to minimize the chance of a type I error). In addition, our findings are best extrapolated to those with chronic or more frequent AF, as, by definition, such subjects would more likely have AF on their baseline ECG. Given our racially heterogenous population, we cannot exclude population stratification as a possible explanation for the associations found between the -174G/C SNP and AF. We had insufficient power (too few outcomes per racial group) to examine these associations for each race and larger studies are required to validate our findings.
In summary, serum IL-6 levels are significantly associated with AF in patients with coronary artery disease. This association is independent of CRP, TNF-α, MCP-1, CD-40 ligand, or fibrinogen and persists after adjusting for multiple potential confounders. The CC genotype of the -174G/C SNP was associated with elevated IL-6 levels in this population, and the same genotype was associated with AF. These findings suggest that IL-6 may play an active role in the pathophysiology of AF rather than simply representing an epiphenomenon related to inflammation. Future studies aimed at investigating the effects of IL-6 on atrial myocardium and AF inducibility will be necessary to determine if this cytokine is indeed active and sufficient to increase the propensity to develop AF.
Acknowledgments
We gratefully acknowledge the assistance of Pui-Yan Kwok, MD, PhD for his participation in and supervision of the genotyping experiments performed for this study and Bradley Aouizerat, PhD for his thoughtful input regarding the genetic epidemiology.
Funding Sources
This work was made possible by Grant Number K12 RR024130 (G.M.M.) from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research, American Heart Association Western States Affiliate Beginning Grant-in-Aid Award (G. M. M.), the National Heart, Lung, and Blood Institute grant RO1-HL072854 (J.E.O.) and NIH. The Heart and Soul Study was funded by grants from the department of Veterans Affairs (Epidemiology Merit Review Program), Washington, DC; the National Heart Lung and Blood Institute grant R01 HL079235, the Robert Wood Johnson Fundation (Generalist Physician Faculty Scholar Program), Princeton, NJ; the American Federation for Aging Research (Paul Beeson Faculty Scholars in Aging Research Program), New York, NY; and the Ischemia Research and Education Foundation, San Bruno, CA.
Footnotes
Disclosures/ conflicts of interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37(2):371–378. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 2.Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003;41(12):2197–2204. doi: 10.1016/s0735-1097(03)00464-9. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100(1):87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 4.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96(4):1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 5.Marcus GM, Yang Y, Varosy PD, et al. Regional left atrial voltage in patients with atrial fibrillation. Heart Rhythm. 2007;4(2):138–144. doi: 10.1016/j.hrthm.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104(24):2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 7.Ellinor PT, Low A, Patton KK, Shea MA, MacRae CA. C-Reactive protein in lone atrial fibrillation. Am J Cardiol. 2006;97(9):1346–1350. doi: 10.1016/j.amjcard.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 8.Morrow DA, Ridker PM. C-reactive protein, inflammation, and coronary risk. Med Clin North Am. 2000;84(1):149–161. doi: 10.1016/s0025-7125(05)70211-x. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–844. [PubMed] [Google Scholar]
- 10.Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8 (Suppl 2):S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conway DS, Buggins P, Hughes E, Lip GY. Relationship of interleukin-6 and C- reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol. 2004;43(11):2075–2082. doi: 10.1016/j.jacc.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 12.Conway DS, Buggins P, Hughes E, Lip GY. Relation of interleukin-6, C-reactive protein, and the prothrombotic state to transesophageal echocardiographic findings in atrial fibrillation. Am J Cardiol. 2004;93(11):1368–1373. A1366. doi: 10.1016/j.amjcard.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 13.Psychari SN, Apostolou TS, Sinos L, Hamodraka E, Liakos G, Kremastinos DT. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am J Cardiol. 2005;95(6):764–767. doi: 10.1016/j.amjcard.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Gaudino M, Andreotti F, Zamparelli R, et al. The -174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation. 2003;108(10 Suppl 1):II195–199. doi: 10.1161/01.cir.0000087441.48566.0d. [DOI] [PubMed] [Google Scholar]
- 15.Bittar MN, Carey JA, Barnard J, et al. Interleukin 6 G-174C polymorphism influences outcome following coronary revascularization surgery. Heart Surg Forum. 2005;8(3):E140–145. doi: 10.1532/HSF98.20041120. discussion E145. [DOI] [PubMed] [Google Scholar]
- 16.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA. 2003;290(2):215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu TM, Kwok PY. Homogeneous primer extension assay with fluorescence polarization detection. Methods Mol Biol. 2003;212:177–187. doi: 10.1385/1-59259-327-5:177. [DOI] [PubMed] [Google Scholar]
- 18.Zaroff JG, Pawlikowska L, Miss JC, et al. Adrenoceptor polymorphisms and the risk of cardiac injury and dysfunction after subarachnoid hemorrhage. Stroke. 2006;37(7):1680–1685. doi: 10.1161/01.STR.0000226461.52423.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conway DS, Buggins P, Hughes E, Lip GY. Prognostic significance of raised plasma levels of interleukin-6 and C-reactive protein in atrial fibrillation. Am Heart J. 2004;148(3):462–466. doi: 10.1016/j.ahj.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Fuster V, Ryden LE, Asinger RW, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): developed in Collaboration With the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol. 2001;38(4):1231–1266. doi: 10.1016/s0735-1097(01)01587-x. [DOI] [PubMed] [Google Scholar]
- 21.Egashira K. Molecular mechanisms mediating inflammation in vascular disease: special reference to monocyte chemoattractant protein-1. Hypertension. 2003;41(3 Pt 2):834–841. doi: 10.1161/01.HYP.0000051642.65283.36. [DOI] [PubMed] [Google Scholar]
- 22.Goser S, Ottl R, Brodner A, et al. Critical role for monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha in induction of experimental autoimmune myocarditis and effective anti-monocyte chemoattractant protein-1 gene therapy. Circulation. 2005;112(22):3400–3407. doi: 10.1161/CIRCULATIONAHA.105.572396. [DOI] [PubMed] [Google Scholar]
- 23.McDermott DH, Yang Q, Kathiresan S, et al. CCL2 polymorphisms are associated with serum monocyte chemoattractant protein-1 levels and myocardial infarction in the Framingham Heart Study. Circulation. 2005;112(8):1113–1120. doi: 10.1161/CIRCULATIONAHA.105.543579. [DOI] [PubMed] [Google Scholar]
- 24.Calabrese F, Carturan E, Chimenti C, et al. Overexpression of tumor necrosis factor (TNF)alpha and TNFalpha receptor I in human viral myocarditis: clinicopathologic correlations. Mod Pathol. 2004;17(9):1108–1118. doi: 10.1038/modpathol.3800158. [DOI] [PubMed] [Google Scholar]
- 25.Satoh M, Nakamura M, Satoh H, Saitoh H, Segawa I, Hiramori K. Expression of tumor necrosis factor-alpha--converting enzyme and tumor necrosis factor-alpha in human myocarditis. J Am Coll Cardiol. 2000;36(4):1288–1294. doi: 10.1016/s0735-1097(00)00827-5. [DOI] [PubMed] [Google Scholar]
- 26.Tuomisto K, Jousilahti P, Sundvall J, Pajunen P, Salomaa V. C-reactive protein, interleukin-6 and tumor necrosis factor alpha as predictors of incident coronary and cardiovascular events and total mortality. A population-based, prospective study. Thromb Haemos. 2006;95(3):511–518. doi: 10.1160/TH05-08-0571. [DOI] [PubMed] [Google Scholar]
- 27.Heeschen C, Dimmeler S, Hamm CW, et al. Soluble CD40 ligand in acute coronary syndromes. N Engl J Med. 2003;348(12):1104–1111. doi: 10.1056/NEJMoa022600. [DOI] [PubMed] [Google Scholar]
- 28.Roldan V, Marin F, Blann AD, et al. Interleukin-6, endothelial activation and thrombogenesis in chronic atrial fibrillation. Eur Heart J. 2003;24(14):1373–1380. doi: 10.1016/s0195-668x(03)00239-2. [DOI] [PubMed] [Google Scholar]
- 29.Luckett LR, Gallucci RM. Interleukin-6 (IL-6) modulates migration and matrix metalloproteinase function in dermal fibroblasts from IL-6KO mice. Br J Dermatol. 2007;156(6):1163–1171. doi: 10.1111/j.1365-2133.2007.07867.x. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Cui G, Esmailian F, et al. Atrial extracellular matrix remodeling and the maintenance of atrial fibrillation. Circulation. 2004;109(3):363–368. doi: 10.1161/01.CIR.0000109495.02213.52. [DOI] [PubMed] [Google Scholar]
- 31.Fishman D, Faulds G, Jeffery R, et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Inves. 1998;102(7):1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terry CF, Loukaci V, Green FR. Cooperative influence of genetic polymorphisms on interleukin 6 transcriptional regulation. J Biol Chem. 2000;275(24):18138–18144. doi: 10.1074/jbc.M000379200. [DOI] [PubMed] [Google Scholar]


