Abstract
The Gram-positive pathogen Group B Streptococcus (GBS) is the leading cause of bacterial pneumonia, sepsis, and meningitis in human newborns. GBS elaborates a pore-forming toxin known as CAMP factor that synergizes with Staphylococcus aureus β-toxin, generating a co-hemolytic reaction useful in identification of GBS in the clinical laboratory. To evaluate the indirect evidence implicating CAMP factor in Group B Streptococcus (GBS) pathogenesis, the cfb gene encoding the pore-forming cytotoxin was deleted by precise allelic replacement. The virulence properties of the CAMP factor mutant were then explored by a series of in vitro and in vivo assays. Compared to wild-type, the isogenic GBS Δcfb mutant demonstrated equivalent phagocyte resistance and endothelial cell invasiveness and also retained full virulence in a mouse model of infection. Our data suggest that CAMP factor expressed in its native context is not essential for systemic virulence of GBS.
Keywords: Bacterial infection, Streptococcus agalactiae, pore-forming toxin, hemolysin
1. Introduction
Group B Streptococcus (GBS) is a major cause of sepsis and meningitis in human neonates. A curious phenomenon of the clinical microbiology laboratory first described by Christie, Atkins and Munch-Peterson in 1944 [1] involves the lysis of sheep red blood cells by the combined action of Staphylococcus aureus β-toxin (sphyngomyelinase) and an extracellular product of GBS, commonly known by the acronym CAMP factor after the discoverers. This CAMP factor co-hemolytic reaction later found utility as a diagnostic tool for presumptive identification of GBS [2, 3]. In 1988, the complete 226 amino acid sequence of GBS CAMP factor was deduced manually from peptide fragments [4] and its encoding gene (cfb) was successfully cloned and expressed in Escherichia coli [5]. Subsequent studies have identified CAMP factor homologues in several other bacterial species including groups A, C and G streptococci, Streptococcus uberis, Actinobacillus pleuropneumoniae, and Propionibacterium acnes [6-9].
Elegant functional analyses indicate that GBS CAMP factor oligomerizes to generate discrete pores in target cells, using the carbohydrate core of GPI-anchored proteins as a membrane receptor [10, 11]. CAMP factor is often proposed as a GBS virulence factor, but published evidence comprises just two studies applying indirect methods: (a) high concentrations of partially purified preparations of the toxin injected intravenously were lethal to mice or rabbits [12], and (b) mice given sublethal doses of GBS and then treated with seven repeated intravenous injections of purified CAMP factor over the next nine hours developed fatal septicemia [13]. To specifically test the role of endogenous CAMP factor production in GBS pathogenicity, we adopted an approach of targeted mutagenesis.
2. Results
2.1 Deletion of cfb gene in GBS
The cfb gene encoding CAMP factor was deleted in well-characterized virulent serotype III GBS strain COH1 by precise, in-frame allelic replacement with a chloramphenicol acetyltransferase (cat) antibiotic resistance marker (Fig. 1A). The isogenic Δcfb mutant lost the ability to generate the co-hemolytic CAMP reaction when streaked adjacent to β-toxin expressing S. aureus on sheep's blood agar (Fig. 1B). Complementation studies showed the CAMP phenotype of the Δcfb mutant could be fully restored by reintroduction the wild-type (WT) cfb gene on expression plasmid pDCerm [14] (Fig. 1B).
Figure 1.
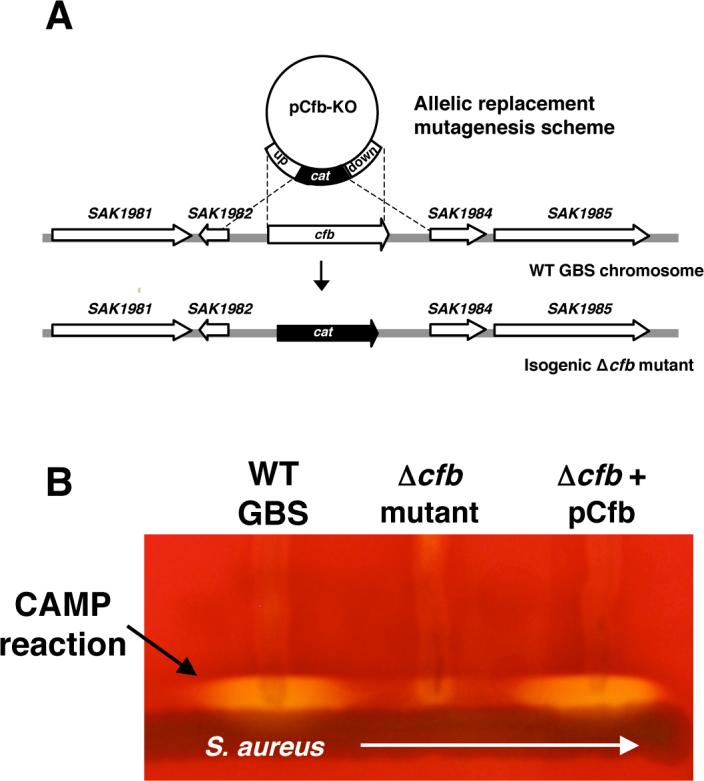
Mutagenesis of the GBS CAMP factor phenotype. (A) Scheme for targeted allelic replacement of the GBS cfb gene (sequences based on genome of GBS strain A909; GenBank CP000114); (B) Loss of co-hemolytic activity of GBS Δcfb mutant when streaked on blood agar media adjacent to β-toxin expressing Staphylococcus aureus; CAMP phenotype is restored upon complementation with cfb gene on a plasmid vector.
2.2 In vitro phenotypic analysis of wild-type and isogenic Δcfb mutant GBS
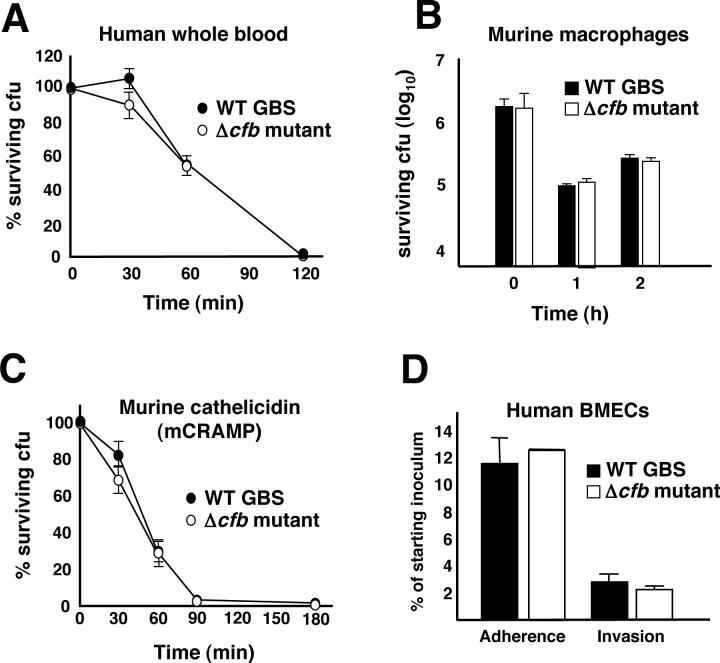
The capacity of GBS to produce systemic infection reflects an ability of the pathogen to resist host innate immune clearance mechanisms. Using published assays [15], we found no differences in the survival kinetics of WT and isogenic Δcfb mutant GBS upon incubation with freshly isolated human whole blood (Fig. 2A) or purified neutrophils (data not shown) from healthy human volunteers. Likewise, no differences were identified in survival of the WT parent strain and Δcfb mutant upon co-incubation with cultured J774 murine macrophages (Fig. 2B). Cathelicidin antimicrobial peptides are important in mammalian defense against invasive streptococcal infection [16], but killing kinetics for murine cathelicidin mCRAMP were similar for WT and Δcfb mutant GBS (Fig. 2C). Invasion of human blood-brain barrier endothelium is a key step in the pathogenesis of GBS meningitis; however, in a standard tissue culture adhesion/invasion assay [17], no differences were observed between the WT and Δcfb mutant in adherence to and invasion of cultured human brain microvascular endothelial cells (hBMEC) (Fig. 2D).
Figure 2.
In vitro analyses of GBS Δcfb mutant. The isogenic GBS Δcfb mutant does not differ from the WT parent strain in susceptibility to (A) human whole blood, (B) murine macrophage, or (C) cathelicidin antimicrobial peptide killing. (D) WT GBS and Δcfb mutant are comparable in adherence to and invasion of cultured human brain microvascular endothelial cells.
2.3. Virulence of wild-type and isogenic Δcfb mutant GBS in mice
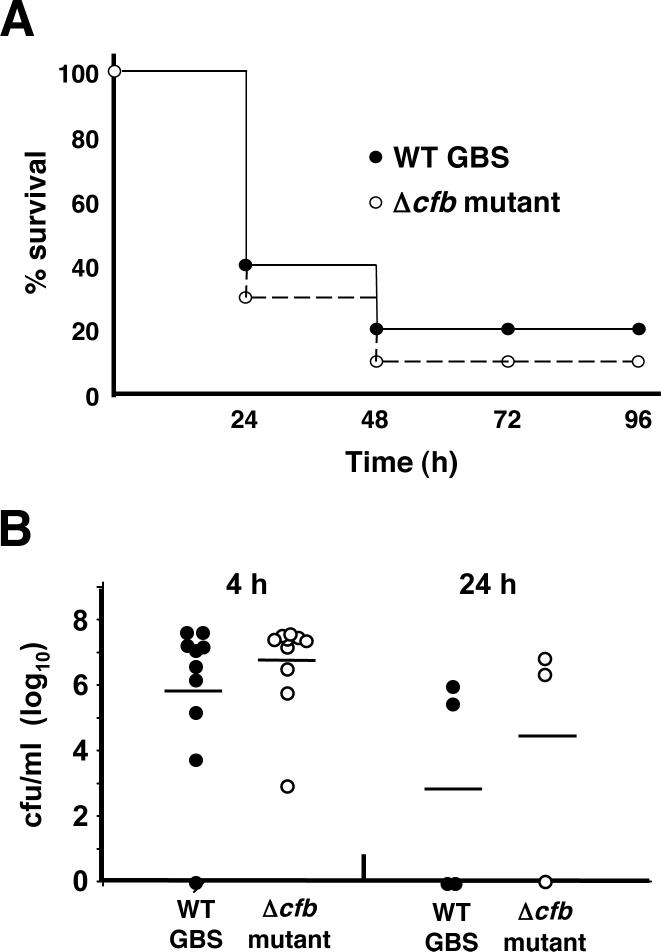
To determine the requirement of GBS CAMP factor production for animal virulence, we challenged 8−10 week old C57Bl/6 mice (n = 10 per group) with 108 cfu of either WT or Δcfb mutant GBS by intraperitoneal injection. Mice were monitored twice daily for mortality, and the Kaplan-Meier survival curves for the two groups were essentially identical (Fig. 3A). Quantitative bacterial cultures from all mice at 4 h and from those surviving at 24 h revealed similar bacterial loads (Fig. 3B).
Figure 3.
CAMP factor is not essential for GBS virulence in mice. Following intraperitoneal challenge of C57Bl/6 mice, WT and isogenic Δcfb mutant GBS produce similar mortality profiles (A) and reach equivalent levels of bacterial counts in the blood (B).
3. Discussion
GBS elaborates a pore-forming β-hemolysin/cytolysin that promotes phagocyte resistance, host cellular invasion, blood-brain barrier penetration, and virulence in mice [15, 18-20]. Tested here using similar in vitro and in vivo model systems, we were unable to differentiate a significant role for the GBS co-hemolysin CAMP factor in these phenotypes. Our data indicate that despite the reported toxicity of CAMP factor preparations [12, 13], the toxin fails to satisfy molecular Koch's postulates [21] as an essential virulence factor in GBS systemic mouse infection.
Immunoglobulin Fc-binding capabilities of purified GBS CAMP factor were previously documented [13], and this phenomenon was originally believed to impact the influence of CAMP factor on septicemia and mortality; however, newer data question the validity of this observation [22]. Nevertheless, conservation of CAMP factor among GBS strains (and in other species listed above) implies an evolutionary benefit of the toxin in the overall context of the bacterium's ecology. Recent evidence reveals expression of the GBS cfb gene is differentially regulated through the CsrRS/CovRS two-component system [23, 24] and the eukaryotic-type serine/threonine kinase Stk1 [25], which control additional phenotypic features of the pathogen including the β-hemolysin/cytolysin toxin. Future analysis of CAMP factor function will be aided by the availability of the isogenic GBS Δcfb knockout mutant described in this report.
4. Materials and Methods
4.1 Bacterial strains
GBS strain COH1 is a well-characterized, highly encapsulated serotype III clinical isolate. Escherichia coli strain MC1061 was used for molecular cloning procedures. GBS strains were grown in Todd-Hewitt broth (THB) or agar (Hardy Diagnostics), and E. coli were grown in Lura-Bertani broth (LB) or agar (Hardy Diagnostics). Were required, antibiotic selection utilized 5 μg/ml erythromycin (Em) or 2 μg/ml chloramphenicol (CM) for GBS and 500 μg/ml Em for E. coli.
4.2. Allelic replacement mutagenesis and complementation
Our established methods of PCR-based targeting vector construction in temperature-sensitive plasmid pHY304 were utilized [17, 26] were utilized. Primer sequences were based on the published genome of GBS strain A909 (GenBank CP000114). Briefly, ∼500 bp of sequence immediately upstream of cfb was from the COH1 chromosome using primers CAMP-UpF 5’-GCTTTTTAAGCGTCTAAACTACTTGGTCTAGTATG-3’ and CAMP-UpR 5’-TAATGAATTTCCTCCTTTAAACTAA–3’. Similarly, ∼500 bp immediately downstream of cfb was amplified from the COH1 chromosome using primers CAMP-DownF 5’-TATGGTAGACTACACAGGACATATTTACACT-3’ and CAMP-DownR 5’-TAATATTTGCATTTTTCGTGTGATGC-3’. The CAMP-UpR and CAMP-DownF primers were constructed with 25-bp 5' extensions corresponding to the 5' and 3' ends of the cat gene, respectively. The amplified PCR products were combined with a 650 bp amplicon of cat in a second round of fusion PCR using the CAMP-UpF and CAMP-DownR primers. The resulting PCR amplicon, containing an in-frame substitution of the cat gene for cfb, was subcloned directly into the temperature-sensitive vector, pHY304. Electroporetic transformation, and sequential identification of single and double crossover events in the GBS chromosome by differential antibiotic and temperature selection were completed as described [17, 26]. Precise in-frame allelic replacement of cfb with cat was confirmed by PCR, sequencing and loss of CAMP reaction phenotype. For complementation of the Δcfb mutant, primers CAMP-UpF and CAMP-DownR were used to amplify the WT cfb factor gene from the COH1 genome. The fragment was directionally cloned into the expression vector pDCerm [14] and used to transform the Δcfb mutant, and complemented strains identified by Em resistance and restriction analysis of the purified plasmid.
4.3 In vitro assays
Whole blood and neutrophil killing assays were performed essentially as described [15]. Briefly, whole blood was drawn from consenting healthy volunteers in accordance with UCSD Institutional Review Board policies. Neutrophils were isolated using the Histopaque cell separation system (Sigma). Overnight cultures of GBS were diluted in THB and grown to mid-logarithmic phase (A600 = 0.4 = ∼108 cfu/ml), washed × 2 and resuspended in PBS, added to the blood or neutrophils at specified inocula, then incubated at 37°C on a rotating platform (for whole blood) or in a 96 well plate in a 5% CO2 incubator (for neutrophils) for up to 3 h, at which point viable GBS cfu were enumerated by plating dilutions on THA. Cultured J774 macrophage were propagated in 24 well plates to ∼1 × 106 cells/well in RPMI +10% FBS. GBS were added at a multiplicity of infection (MOI) = 0.1 bacteria/cell and incubated for 1 h at 37°C. Monolayers were washed × 3, media containing gentamicin (100 μg/ml) and penicillin (5 μg/ml) added for 2 h to kill extracellular bacteria, monolayers washed × 3 again, cells lysed with triton X-100 and serial dilutions plated for enumeration of surviving cfu. Bacterial killing by the antimicrobial peptide, CRAMP, was performed essentially as described [16]. Briefly, 6 × 104 cfu bacteria were incubated in a total volume of 200 μl in individual wells of a microtiter plates at 37°C. 8uM of CRAMP was added to each reaction well. Samples were drawn at various time points, and serial dilutions were plated to quantify cfu. The ability of GBS to adhere to and invade cultured human brain microvascular endothelial cells was performed as previously described [19].
4.3 In vivo mouse infection model
A murine model of systemic infection was used to assess the virulence of the CAMP factor mutant GBS. The in vivo protocol was approved by the University of California, San Diego Animal Subjects Committee. For these studies dilutions of overnight cultures of wild type and Δcfb COH1 GBS strains were grown to mid-logarithmic phase. Eight to ten week old C57BL/6 mice (n = 10 per group) were then injected intraperitoneally with 108 cfu of either bacterial strain and were monitored twice daily for three days for mortality. Blood was collected at 4 and 24 hours for enumeration of bacterial load. Survival was monitored to 4 days after inoculation. Animals appearing moribund during the study were humanely euthanized.
5. Acknowledgements
This work was supported by an American Heart Association Established Investigator Award and American Lung Association Career Investigator Award (VN) and NIH Grants HD051796 (VN) and NS051247 (KSD).
Abbreviations used
- GBS
Group B Streptococcus
- THB
Todd-Hewitt broth
- THA
Todd-Hewitt agar
- LB
Luria-Bertani broth
- cfu
colony-forming units
- MOI
multiplicity of infection
- cat
chloramphenicol acetyltransferase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- 1.Christie R, Atkins NE, Munch-Petersen E. A note on a lytic phenomenon shown by group B streptococci. Aust J Exp Biol Med Sci. 1944;22:197–200. doi: 10.1038/icb.1945.30. [DOI] [PubMed] [Google Scholar]
- 2.Phillips EA, Tapsall JW, Smith DD. Rapid tube CAMP test for identification of Streptococcus agalactiae (Lancefield group B). J Clin Microbiol. 1980;12:135–7. doi: 10.1128/jcm.12.2.135-137.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapsall JW, Phillips EA. Presumptive identification of group B streptococci by rapid detection of CAMP factor and pigment production. Diagn Microbiol Infect Dis. 1987;7:225–8. doi: 10.1016/0732-8893(87)90010-1. [DOI] [PubMed] [Google Scholar]
- 4.Ruhlmann J, Wittmann-Liebold B, Jurgens D, Fehrenbach FJ. Complete amino acid sequence of protein B. FEBS Lett. 1988;235:262–6. doi: 10.1016/0014-5793(88)81275-4. [DOI] [PubMed] [Google Scholar]
- 5.Schneewind O, Friedrich K, Lutticken R. Cloning and expression of the CAMP factor of group B streptococci in Escherichia coli. Infect Immun. 1988;56:2174–9. doi: 10.1128/iai.56.8.2174-2179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey J, Perrin J, Nicolet J. Cloning and expression of a cohemolysin, the CAMP factor of Actinobacillus pleuropneumoniae. Infect Immun. 1989;57:2050–6. doi: 10.1128/iai.57.7.2050-2056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopes MF, Merquior VL, Peralta JM, Teixeira LM. Partial characterization of the cohemolytic factor produced by Streptococcus uberis and comparison with the CAMP-factor. FEMS Immunol Med Microbiol. 1995;12:205–12. doi: 10.1111/j.1574-695X.1995.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 8.Gase K, Ferretti JJ, Primeaux C, McShan WM. Identification, cloning, and expression of the CAMP factor gene (cfa) of group A streptococci. Infect Immun. 1999;67:4725–31. doi: 10.1128/iai.67.9.4725-4731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valanne S, McDowell A, Ramage G, Tunney MM, Einarsson GG, O'Hagan S, et al. CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiology. 2005;151:1369–79. doi: 10.1099/mic.0.27788-0. [DOI] [PubMed] [Google Scholar]
- 10.Lang S, Palmer M. Characterization of Streptococcus agalactiae CAMP factor as a pore-forming toxin. J Biol Chem. 2003;278:38167–73. doi: 10.1074/jbc.M303544200. [DOI] [PubMed] [Google Scholar]
- 11.Lang S, Xue J, Guo Z, Palmer M. Streptococcus agalactiae CAMP factor binds to GPI-anchored proteins. Med Microbiol Immunol. 2007;196:1–10. doi: 10.1007/s00430-006-0021-2. [DOI] [PubMed] [Google Scholar]
- 12.Skalka B, Smola J. Lethal effect of CAMP-factor and UBERIS-factor--a new finding about diffusible exosubstances of Streptococcus agalactiae and Streptococcus uberis. Zentralbl Bakteriol. 1981;249:190–4. [PubMed] [Google Scholar]
- 13.Jurgens D, Sterzik B, Fehrenbach FJ. Unspecific binding of group B streptococcal cocytolysin (CAMP factor) to immunoglobulins and its possible role in pathogenicity. J Exp Med. 1987;165:720–32. doi: 10.1084/jem.165.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeng A, Sakota V, Li Z, Datta V, Beall B, Nizet V. Molecular genetic analysis of a group A Streptococcus operon encoding serum opacity factor and a novel fibronectin-binding protein, SfbX. J Bacteriol. 2003;185:1208–17. doi: 10.1128/JB.185.4.1208-1217.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu GY, Doran KS, Lawrence T, Turkson N, Puliti M, Tissi L, et al. Sword and shield: linked group B streptococcal β-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci U S A. 2004;101:14491–6. doi: 10.1073/pnas.0406143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nizet V, Ohtake T, Lauth X, Trowbridge J, Rudisill J, Dorschner RA, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 17.Doran KS, Engelson EJ, Khosravi A, Maisey HC, Fedtke I, Equils O, et al. Blood-brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J Clin Invest. 2005;115:2499–507. doi: 10.1172/JCI23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doran KS, Chang JC, Benoit VM, Eckmann L, Nizet V. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J Infect Dis. 2002;185:196–203. doi: 10.1086/338475. [DOI] [PubMed] [Google Scholar]
- 19.Doran KS, Liu GY, Nizet V. Group B streptococcal β-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J Clin Invest. 2003;112:736–44. doi: 10.1172/JCI17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puliti M, Nizet V, von Hunolstein C, Bistoni F, Mosci P, Orefici G, et al. Severity of group B streptococcal arthritis is correlated with beta-hemolysin expression. The J Infect Dis. 2000;182:824–32. doi: 10.1086/315773. [DOI] [PubMed] [Google Scholar]
- 21.Falkow S. Molecular Koch's postulates applied to microbial pathogenicity. Rev Infect Dis. 1988;10(Suppl 2):S274–6. doi: 10.1093/cid/10.supplement_2.s274. [DOI] [PubMed] [Google Scholar]
- 22.El-Huneidi W, Mui R, Zhang TH, Palmer M. Streptococcus agalactiae CAMP factor/protein B does not bind to human IgG. Med Microbiol Immunol. 2007;196:73–7. doi: 10.1007/s00430-006-0028-8. [DOI] [PubMed] [Google Scholar]
- 23.Jiang SM, Cieslewicz MJ, Kasper DL, Wessels MR. Regulation of virulence by a two-component system in group B streptococcus. J Bacteriol. 2005;187:1105–13. doi: 10.1128/JB.187.3.1105-1113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamy MC, Zouine M, Fert J, Vergassola M, Couve E, Pellegrini E, et al. CovS/CovR of group B streptococcus: a two-component global regulatory system involved in virulence. Mol Microbiol. 2004;54:1250–68. doi: 10.1111/j.1365-2958.2004.04365.x. [DOI] [PubMed] [Google Scholar]
- 25.Rajagopal L, Vo A, Silvestroni A, Rubens CE. Regulation of cytotoxin expression by converging eukaryotic-type and two-component signalling mechanisms in Streptococcus agalactiae. Mol Microbiol. 2006;62:941–57. doi: 10.1111/j.1365-2958.2006.05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis AL, Nizet V, Varki A. Discovery and characterization of sialic acid O-acetylation in group B Streptococcus. Proc Natl Acad Sci U S A. 2004;101:11123–8. doi: 10.1073/pnas.0403010101. [DOI] [PMC free article] [PubMed] [Google Scholar]