Abstract
BACKGROUND
Atrial arrhythmias are associated with inflammation. The cause and effect of the association is unknown.
OBJECTIVE
To test the hypothesis that atrial tachyarrhythmias contribute to inflammation.
METHODS
We performed a prospective observational study wherein C-Reactive Protein (CRP) and interleukin-6 (IL-6) levels from the femoral vein and coronary sinus (CS) were compared prior to curative ablation for atrial flutter (AFL, n=59) and paroxysmal supraventricular tachycardia (SVT, n=110). Follow-up levels were obtained at 1 and 6 months.
RESULTS
Peripheral levels of both biomarkers were significantly higher in the AFL group. After multivariate adjustment, only those in the AFL group who presented in AFL or atrial fibrillation (AF) had significantly elevated CRP levels (OR 1.26, p=0.033). Levels of each marker were similar in the CS and peripheral blood in the SVT group; in the AFL group, both CRP and IL-6 were significantly lower in the CS than the periphery (p=0.0076 and p=0.0021, respectively). CRP was significantly lower a median of 47 days after AFL ablation (from a median 6.28 mg/L to 2.92 mg/L, p=0.028) and remained reduced at second follow-up. IL-6 decreased across three time points after AFL ablation (p=0.002). No reduction in inflammatory biomarkers was observed after SVT ablation.
CONCLUSIONS
CRP and IL-6 levels are elevated in patients presenting in AFL. Given the lower CS values in these patients, their origin appears to be systemic rather than cardiac. Because these levels significantly fall after ablation of AFL, the atrial tachyarrhythmia appears to be the cause (not the effect) of the inflammation.
Keywords: Atrial arrhythmias, Atrial flutter, Inflammation, CRP, IL-6, Ablation
Introduction
Although atrial arrhythmias are the most common arrhythmias seen in clinical practice, the exact etiology remains unknown.1 Recent data suggest that inflammation may play an important role,2 but the cause and effect nature of this relationship is not known (does the rhythm cause the inflammation or the inflammation cause the rhythm?). The majority of studies examining this relationship have exclusively or predominately enrolled patients with atrial fibrillation (AF).3 Atrial flutter (AFL) is often observed in patients with AF, is often seen in patients with the same disease states and risk factors as AF, carries similar risks of thromboembolic complications, and, like AF, involves prolonged periods of markedly elevated atrial rates.3,4 Given the high success rate of typical AFL ablation,5–7 this particular arrhythmia provides the opportunity to measure inflammatory markers before and after the arrhythmia is cured.
With the hypothesis that atrial tachyarrhythmias contribute to inflammation, we performed a prospective analysis of patients with typical AFL in order to assess biomarkers of inflammation before and after ablation procedures.
Methods
Participants
Consecutive adult patients presenting for ablation of symptomatic typical AFL over a 2 year period were enrolled at a single university medical center. The diagnosis of typical AFL was made based on the 12 lead surface electrocardiogram, requiring the following characteristics: an atrial rate of 250–350 beats per minute8 with either positive flutter waves in lead V1 and negative flutter waves in the inferior limb leads or negative flutter waves in lead V1 and positive flutter waves in the inferior limb leads, and a transition in the orientation of the flutter waves across the precordium.9 Over the same time period, consecutive patients presenting for ablation of paroxysmal supraventricular tachycardia (SVT) were enrolled as controls. For the SVT group, either documented paroxysmal narrow complex tachycardia or symptoms and a clinical suspicion by the treating physician of SVT were adequate. Patients were excluded from the SVT group for incessant tachycardia (any history of tachycardia lasting > 24 hours) or any previous history of AFL or AF.
Patients were excluded from either group if they were likely to have elevated serologic markers of inflammation independent of the association with their arrhythmia: patients with a history of a myocardial infarction or elevated troponin levels within the previous 3 months, major trauma or surgery within the previous 3 months, any chronic inflammatory disease (including chronic rheumatologic diseases requiring immunosuppressive agents), chronic infectious diseases requiring treatment, any active malignancy, any acute rheumatologic or infectious disease (including symptoms of a common upper respiratory tract infection), or any other condition that would be expected to cause a fever, elevated white blood cell count, or elevated erythrocyte sedimentation rate. Those who might have been unable to produce elevated serologic markers were also excluded: patients on immunosuppressive therapy (e.g., steroids), and those with leukopenia of any etiology. Patients with congenital heart disease (corrected or not) were also excluded.
All patients provided witnessed and written informed consent. The study was approved by the UCSF Committee on Human Research.
Markers of inflammation
After administration of intravenous fentanyl and midazolam for conscious sedation, each patient underwent placement of intravenous sheaths in the right internal jugular vein and at least one of the femoral veins. All patients had standard electrode catheters placed for electrophysiology study, including a coronary sinus catheter, a His catheter, and an ablation catheter; all AFL patients had a multipolar catheter placed around the tricuspid annulus and all SVT patients had an electrode catheter placed in the right ventricle. After the sheaths and catheters had been placed but before any pacing or ablation was performed, a total of 40 cc of blood was obtained for study purposes. After discarding 10 cc of blood, blood was withdrawn from both the right femoral sheath and a luminal coronary sinus catheter. Blood was confirmed to arise from the coronary sinus based on 3 criteria: the fluoroscopic image of the catheter in standard right anterior oblique and left anterior oblique projections, intracardiac electrograms consistent with coronary sinus placement, and the gross appearance of blood as darker than peripheral blood withdrawn from the femoral sheath.
Patients were asked to return for a first follow-up visit at approximately 1 month after the procedure and a second follow-up visit approximately 6 months after the procedure. During each of those visits, the interim clinical history was reviewed, a 12 lead electrocardiogram was obtained, and blood was obtained from a peripheral vein.
All blood obtained was centrifuged and serum aliquoted and stored in a −80 degree Celsius freezer. High-sensitivity C-Reactive Protein (CRP) was determined by ELISA (Alpha Diagnostic International, San Antonio, TX) and high-sensitivity interleukin-6 (IL-6) levels were determined by ELISA (R & D systems, Minneapolis, MN). The lower limit of detection for CRP was 0.00035 mg/L and the lower limit of detection for IL-6 was 0.447 pg/ml.
Statistical Analysis
Normally distributed continuous variables are expressed as means ± SD; continuous variables not normally distributed are presented as medians and interquartile ranges (IQR). Bivariate analyses of normally distributed continuous variables are assessed using t-tests and categorical variables were compared using the χ2 test. Consistent with previous studies, IL-610,11 and CRP2 were right-skewed. Bivariate analysis of these biomarkers across groups was therefore performed using the Wilcoxon rank sum test; bivariate analyses within groups (eg, before and after ablation in a given study group) was performed using the paired t-tests and Wilcoxon signed rank test as appropriate. Spearman’s rank correlation coefficient was used to assess for correlations and the Kruskal–Wallis rank test was used to assess overall differences in markers across multiple categories (eg, race). The Wald test for trend was employed to assess for significant linear trends. Multivariable analysis was performed with logistic regression analysis, and covariates/potential confounders were selected based on both important demographics (eg, age and gender) and those covariates significantly associated with both the predictors and outcomes of interest with p values <0.10. Otherwise, two-tailed p vales < 0.05 were considered significant.
Results
One hundred and sixty-nine patients were enrolled, 59 with AFL and 110 with SVT. Baseline clinical characteristics are listed in Table 1. Patients in the AFL group were older, more often men and more often with hypertension, coronary artery disease, and congestive heart failure. Of those with AFL, 26 (44%) had a clinical history of AF. AFL patients with and without a history of AF differed only in statin use (58% of those with AF versus 30% of those without AF, p=0.035). None of the other covariates (listed in Table 1.) differed between AFL patients with and without AF.
Table 1.
Baseline characteristics of atrial flutter and supraventricular tachycardia patients
| AFL n=59 | SVT n=110 | p value | AFL presenting in AF/AFL n=26 | AFL presenting in sinus n=33 | p value | |
|---|---|---|---|---|---|---|
| Race | ||||||
| White | 43 (73%) | 72 (66%) | 23 (77%) | 20 (70%) | ||
| Black | 2 (3%) | 3 (3%) | 2 (6%) | 0 | ||
| Asian | 9 (15%) | 14 (13%) | 5 (15%) | 4 (15%) | ||
| Latino | 3 (5%) | 14 (13%) | 0.72 | 2 (6%) | 1 (4%) | 0.57 |
| Age (years) | 61 ± 15 | 47 ± 17 | <0.0001 | 64 ± 15 | 57 ± 14 | 0.073 |
| Male | 45 (76%) | 49 (44%) | <0.001 | 25 (76%) | 20 (77%) | 0.92 |
| Body Mass Index (kg/m2) | 29 ± 6 | 27 ± 7 | 0.045 | 29 ± 6 | 29 ± 7 | 0.72 |
| Hypertension | 26 (44%) | 24 (21%) | 0.002 | 14 (42%) | 12 (46%) | 0.78 |
| Type II diabetes | 9 (15%) | 9 (8%) | 0.14 | 6 (18%) | 3 (12%) | 0.48 |
| Coronary artery disease | 11 (19%) | 5 (5%) | 0.002 | 8 (25%) | 3 (12%) | 0.21 |
| Congestive heart failure | 7 (12%) | 0 | <0.001 | 3 (9%) | 4 (15%) | 0.46 |
| *Ejection fraction (%) | 62 ± 5 | 58 ± 8 | 0.026 | 58 ± 10 | 59 ± 5 | 0.76 |
| † Creatinine >1.5 mg/dL | 2 (4%) | 0 | 0.05 | 0 | 2 (8%) | 0.11 |
| Statin use | 25 (42%) | 19 (17%) | <0.001 | 12 (36%) | 13 (50%) | 0.29 |
| ACE inhibitor or ARB | 16 (27%) | 6 (5%) | <0.001 | 10 (30%) | 6 (23%) | 0.54 |
| Aspirin | 26 (44%) | 17 (15%) | <0.001 | 16 (48%) | 10 (39%) | 0.44 |
Transthoracic echocardiograms were available for 30 atrial flutter patients and 36 supraventricular tachycardia patients
None of the patients had renal disease requiring dialysis therapy
AF denotes atrial fibrillation; AFL denotes atrial flutter; AF/AFL denotes either AF or AFL (one patient in this group had AF); SVT denoted supraventricular tachycardia; ARB denotes angiotensin receptor blocker
AFL was first diagnosed a median 189 days prior to the procedure (interquartile range [IQR] 68-1051 days). Twenty-six of the AFL patients were in normal sinus rhythm at the beginning of the procedure (and therefore at the time the initial serum specimen was obtained), 1 was in AF, and 32 were in AFL. During EP study, the mean atrial cycle length of the AFL patients was 256 ± 32 ms, with a mean ventricular cycle length of 358 ± 79 ms. All of the SVT patients were in normal sinus rhythm at the time of the blood draw. Of the SVT patients, the primary diagnosis was typical AV nodal reentrant tachycardia (AVNRT) in 49 (45%), atrioventricular reciprocating tachycardia (AVRT) in 32 (29%), focal atrial tachycardia in 17 (16%), atypical AVNRT in 5 (5%) and sinus rhythm (no inducible sustained arrhythmia) in 7 (6%). The mean atrial and ventricular cycle lengths for the SVTs observed were 358 ± 79 ms and 362 ± 78 ms, respectively.
Baseline Inflammatory Markers
Association between each inflammatory biomarker and clinical characteristics is shown in Table 2. Covariates for the logistic regression model were based on associations with both inflammatory markers and AFL (as shown in Tables 1 and 2) as well as race, gender, and diabetes for “face value.”
Table 2.
Associations between each inflammatory marker and clinical characteristics
| hsCRP | hsIL-6 | |||||
|---|---|---|---|---|---|---|
| rho | p value | rho | p value | |||
| Age (years) | 0.26 | 0.0009 | 0.32 | <0.0001 | ||
| Ejection fraction (%) | −0.174 | 0.89 | −0.21 | 0.098 | ||
| BMI (kg/m2) | 0.44 | <0.0001 | 0.28 | 0.0003 | ||
|
| ||||||
| hsCRP (mg/dL) | p value | hsIL-6 (pg/ml) | p value | |||
|
| ||||||
| Race | ||||||
| White | 3.1 (1.2–6.9) | 2.3 (1.2–4.6) | ||||
| Black | 5.5 (2.7–10.8) | 5.0 (3.3–8.9) | ||||
| Asian | 2.1 (0.9–3.4) | 1.6 (0.8–3.0) | ||||
| Latino | 2.9 (1.1–4.6) | 0.23 | 2.2 (1.2–3.3) | 0.30 | ||
|
| ||||||
| With covariate | Without covariate | p value | With covariate | Without covariate | p value | |
|
| ||||||
| Male | 3.0 (1.2–7.1) | 2.7 (1.1–6.4) | 0.72 | 2.3 (1.1–4.8) | 2.1 (0.9–3.6) | 0.43 |
| Hypertension | 5.7 (2.3–8.4) | 2.3 (0.9–5.1) | 0.0004 | 2.6 (1.7–6.5) | 2.0 (0.8–4.3) | 0.0064 |
| Type II diabetes | 3.3 (2.2–8.4) | 2.7 (1.1–6.4) | 0.29 | 3.3 (1.8–8.9) | 2.2 (1.0–4.3) | 0.062 |
| Coronary artery disease | 2.3 (1.6–8.8) | 2.9 (1.1–6.4) | 0.83 | 3.9 (2.1–9.7) | 2.1 (0.9–4.2) | 0.0066 |
| Congestive heart failure | 8.4 (4.1–10.0) | 2.7 (1.1–6.3) | 0.016 | 5.0 (2.3–16.2) | 2.2 (1.0–4.3) | 0.028 |
| Creatinine>1.5 mg/dL | 7.6 (6.4–8.9) | 2.7 (1.1–6.4) | 0.15 | 24.6 (1.7–47.5) | 2.3 (1.1–4.5) | 0.36 |
| Statin | 3.5 (2.1–8.4) | 2.6 (0.9–5.9) | 0.065 | 2.8 (1.7–5.5) | 2.0 (0.9–3.8) | 0.013 |
| ACE inhibitor or ARB | 4.8 (1.5–10.0) | 2.7 (1.1–6.4) | 0.29 | 5.4 (2.7–10.1) | 2.05 (0.9–3.6) | 0.001 |
| Aspirin | 3.4 (1.6–6.4) | 2.5 (1.1–6.4) | 0.38 | 2.3 (1.7–3.7) | 2.2 (0.9–4.6) | 0.44 |
Interquartile ranges are given in parentheses
ARB denotes angiotensin receptor blocker
Differences in inflammatory markers between AFL and SVT patients as well as AFL patients compared by rhythm at presentation are shown in Figure 1. Of note, levels of inflammatory marker did not differ by primary diagnosis in the SVT group (p=0.64 for CRP and p=0.31 for IL-6). After multiple logistic regression analysis controlling for age, gender, race, hypertension, congestive heart failure, and body mass index (BMI), CRP did not remain significantly elevated in the AFL group (odds ratio [OR] 1.04, 95% confidence interval [CI] 0.96–1.12, p=0.31). Similarly, after adjusting for age, gender, race, hypertension, congestive heart failure, BMI, statin use and ACE inhibitor or angiotensin receptor blocker (ARB) use, IL-6 did not remain significantly associated with AFL (OR 0.99, 95% CI 0.92–1.08, p=0.27).
Figure 1.
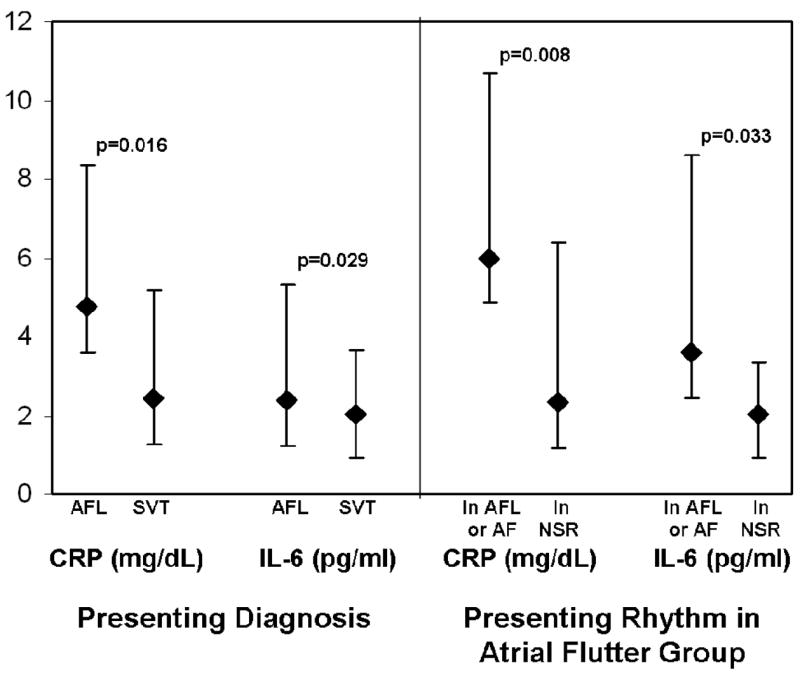
Baseline serologic markers of inflammation (left) in those with atrial flutter (AFL) and supraventricular tachycardia (SVT). On the right, AFL patients that presented in AFL (n=32) or atrial fibrillation (AF, n=1) are compared to the AFL patients that presented in normal sinus rhythm (NSR, n=26). Y error bars denote interquartile ranges.
In contrast, after adjusting for the same covariates in the logistic regression model, CRP remained significantly elevated in those presenting in AFL or AF compared to those in the AFL group that presented in sinus rhythm: for every 1 mg/L unit increase in CRP, there was a 1.26 greater odds (or 26% greater likelihood) of an AFL patient presenting in AFL or AF than sinus rhythm (95% CI 1.02–1.57, p=0.033); similarly, after the same adjustment, those AFL patients presenting in AFL or AF had a significantly higher CRP than patients with SVT (OR 1.1, 95% CI 1.01–1.20, p=0.025). IL-6 levels did not maintain a significant association in those presenting in AFL or AF after adjustment for covariates, but the comparison between those presenting in AFL or AF to the SVT patients neared statistical significance (OR 1.10, 95% CI 1.00–1.22, p=0.058).
Inflammatory Markers in the Coronary Sinus
Thirty-seven (63%) of the AFL patients and 59 (54%) of the SVT patients had coronary sinus blood drawn. The remainder did not have CS blood drawn because they did not have CS access with a luminal catheter (n=24) or because blood could not be withdrawn from the luminal catheter (attributed to the tip lying against the vessel wall). For each group, there were no significant differences in age, gender, race, BMI, EF, presence of hypertension, heart failure, coronary disease, renal insufficiency, statin use, or ACE inhibitor or ARB use between those with and without coronary sinus blood. For the AFL group, the baseline CRP and IL-6 levels also did not differ between those with and without coronary sinus blood (p=0.60 and p=0.90, respectively); baseline CRP and IL-6 levels also did not differ between those with and without coronary sinus blood in the SVT group (p=0.96 and p=0.20, respectively).
Whereas SVT patients exhibited similar femoral and coronary sinus levels of CRP and IL-6, both inflammatory markers were significantly lower in the coronary sinus in those AFL subjects with coronary sinus blood available (Figure 2). Of note, this significant difference was driven by those in AFL or AF at presentation: the lower levels of inflammatory markers in coronary sinus blood remained significant when those in AFL or AF (n=19 with coronary sinus blood) were analyzed alone ( p=0.016 for CRP and p=0.011 for IL-6), but AFL patients presenting in sinus rhythm (n=18 with coronary sinus blood) had no significant differences in femoral versus peripheral levels (p=0.29 for CRP and p=0.12 for IL-6).
Figure 2.
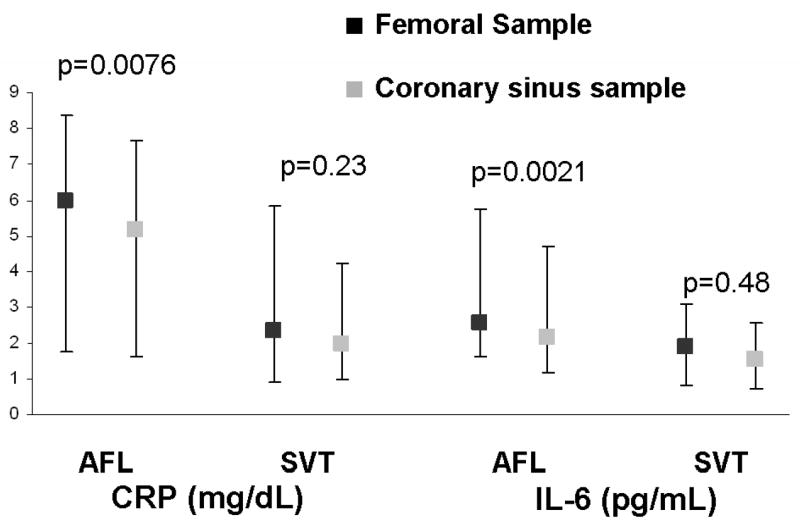
Levels of CRP and IL-6 are each significantly lower in the coronary sinus than the femoral vein in the atrial flutter (AFL) patients (n=37); no significant differences are seen in the supraventricular tachycardia (SVT) patients (n=59). Y error bars denote interquartile ranges.
Inflammatory Markers after Ablation Therapy
Serum was obtained from 26 (44%) of the AFL patients who returned for their first follow-up visit a median of 48 days (IQR 32-77) after the ablation procedure. Of the SVT patients, serum was obtained from 36 (33%) who returned a median 47 days (IQR 30-73) after the ablation procedure. Time to follow up was not significantly different between groups (p=0.62). For each group, there were no significant differences in age, gender, race, BMI, EF, presence of hypertension, heart failure, coronary disease, renal insufficiency, or statin use between those with and without a first follow-up visit. The only difference between those who did and did not have first follow-up was that, in the AFL group only, a greater proportion of those without follow up (39%) used an ACE inhibitor or an ARB compared to those who did follow up (11%, p=0.017). For the AFL group, the baseline CRP and IL-6 levels also did not differ between those with and without first follow-up (p=0.064 and p=0.68, respectively); baseline CRP and IL-6 levels also did not differ between those with and without first follow up in the SVT group (p=0.38 and p=0.91, respectively). Statin use did not change significantly with follow-up: one patient in the AFL that denied statin use at baseline reported statin use at first follow-up. Whereas CRP levels were measured for every follow-up visit, IL-6 was measured in 22 of the 26 with AFL and 32 of the 36 with SVT.
All of the AFL patients who returned for follow up were found to have typical cavotricuspid isthmus dependent flutter during their procedure, and all underwent a successful cavotricuspid isthmus ablation with demonstrated bidirectional block. At the first follow-up visit, one patient exhibited what appeared to be typical AFL by 12 lead electrocardiogram. The remainder presented in sinus rhythm, and none of these patients had interim AFL or AF by history or electrocardiogram. Of the SVT patients with first follow up, all but 2 had successful ablation procedures: one patient with a posteroseptal accessory pathway and inducible orthodromic AVRT and another with a para-Hisian atrial tachycardia. All SVT patients were in sinus rhythm at the time of first follow-up.
Eight patients in the AFL group returned for a second follow-up visit a median 180 days (IQR 125-230) after their ablation procedure, and 7 patients in the SVT group returned for a second follow-up 163 days (IQR 101-275) after their procedure. All of these patients were in sinus rhythm at the time of second follow-up.
In the atrial flutter group, the ventricular rate decreased from a mean 77 ± 22 beats per minute at baseline to a mean 66 ± 12 beats per minute at the first follow-up visit (p=0.019). In the SVT group, the ventricular rate did not significantly change: the baseline heart rate in this group was 76 ± 19 beats per minute at baseline and 76 ± 20 beats per minute at first follow-up (p=0.98).
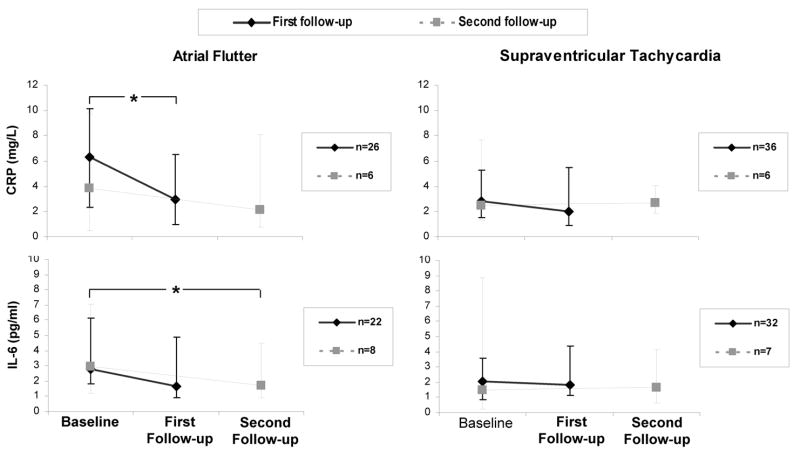
After ablation, CRP decreased significantly in the AFL group and did not change in the SVT group (Figure 3). Although IL-6 did not significantly decrease at the first follow-up, there was a significant decrease directly comparing baseline to the second follow-up levels. In addition, a significant decrease in IL-6 levels across the three time points in the AFL group was observed (p=0.002). None of the comparisons revealed a decrease in either inflammatory marker in the SVT group. After ablation, both CRP and IL-6 levels became similar (with no significant differences) comparing AFL to SVT patients at both first follow-up and second follow-up. A sensitivity analysis was performed including and excluding the patients without successful ablation and did not meaningfully affect the results. The single patient with an unsuccessful AFL ablation exhibited a persistently elevated CRP (18.42 to 16.11 mg/L) and no meaningful change in IL-6 (1.8 pg/ml to 1.7 pg/ml).
Figure 3.
Change in each marker after ablation in the atrial flutter group and the supraventricular tachycardia group. Data for those with first follow-up is shown in solid lines, and data for those with second follow-up is shown in dashed lines. All subjects with second follow-up also had first follow-up. One subject in the AFL group developed a recurrence of AFL (included in the first follow-up data). The asterix (*) denotes a significant decline in marker level with p<0.05. Y error bars denote interquartile ranges.
The reduction in inflammatory markers from baseline to first follow up after ablation in the AFL group was driven by those in AFL or AF during the baseline blood draw: 17 AFL patients with first follow up were in AFL or AF at presentation, and 12 of these had a reduction in their CRP (p=0.0495). Of the 9 AFL patients with first follow up who initially presented in sinus rhythm, CRP rose in 5 and fell in 4 (p=0.52 for a difference between baseline and first follow up). Although not statistically significant (potentially due to the smaller numbers with follow up IL-6 levels), 10 of 14 of the AFL patients with first follow-up IL-6 who initially presented in AFL or AF had a reduction in their IL-6 levels (p=0.25), while IL-6 rose in 4 and fell in 4 in the AFL patients presenting in sinus rhythm (p=0.89 for a difference in IL-6 levels between baseline and first follow-up).
Discussion
This is the first study to demonstrate evidence of inflammation in AFL. Perhaps more importantly, as the first study to demonstrate a reduction in inflammatory markers after definitive treatment for a rapid atrial arrhythmia, our findings suggest that rapid atrial rates contribute to inflammation.
We found markers of inflammation to be significantly elevated in AFL patients. However, only patients with a history of AFL who had their blood drawn while in either AFL or AF exhibited significantly elevated CRP after adjustment for multiple potential confounders. Importantly, those presenting in AFL or AF had significantly elevated CRP after adjustment in comparison to both those with SVT and in comparison to those with a history of AFL who were in a normal rhythm at the time blood was drawn. Coronary sinus levels of both CRP and IL-6 were significantly lower in the AFL patients, and this difference appeared to be driven by those presenting in AFL or AF: this suggests that the peripheral elevations in these markers observed may not be due to a generation of these markers in the heart. Finally, both CRP and IL-6 levels significantly decreased after ablation of AFL, with levels after AFL ablation comparable to those seen in controls. This suggests that the elevation in inflammatory markers seen in the AFL patients is, at least in part, caused by the arrhythmia itself.
Atrial arrhythmias have been shown to be associated with inflammation, but these studies have focused primarily on AF: atrial biopsies of lone AF patients have exhibited histologic findings consistent with active or previous myocarditis12 and CRP has been shown to be associated with AF.2 However, the cause and effect nature of this relationship has yet to be fully elucidated. In some forms of AF, underlying myocardial inflammation or atrial fibrosis is likely the primary cause.13,14 However, before this pathophysiologic model is attributed to all AF or as a satisfactory explanation for the cause and effect relationship between inflammation and AF, the possibility that elevated atrial rates contribute to inflammation must be considered.
One study provided intriguing evidence that AF is preceded by an elevation in CRP,15 but the possibility of previously existing asymptomatic or undiagnosed AF in those patients cannot be excluded. In fact, the inflammation-rapid atrial arrhythmia relationship may represent a “vicious cycle,” potentially offering another explanation for the progressive nature of AF. Two previous studies failed to demonstrate a reduction in CRP after cardioversion of AF, but each was potentially limited by small sample size: one involved only 15 patients with a follow up of 2 weeks16 and the other involved 14 patients (of 25) who remained in sinus rhythm with a follow up of approximately 33 days.17 The absence of a decrease in CRP is not sufficient to exclude the possibility that the arrhythmia contributes to inflammation in these small studies. Furthermore, as cardioversion is associated with high recurrence rates18 and as AF can often be asymptomatic,19 recurrences may have been undetected.
AFL provides an excellent model to study the cause and effect relationship since it is associated with a persistent elevation of severely elevated atrial rates and ablation procedures are highly successful at curing the arrhythmia. With recent ablation tools and accurate endpoints for assessing bidirectional isthmus block, the long-term success rate in preventing recurrent atrial flutter is estimated to be approximately 97%.20 Patients with SVT were used as the control group for 3 reasons: first, as patients referred to the same referral center for procedures at the same electrophysiology laboratory as the study patients, they reflected the same study base; second, we were able to obtain coronary sinus blood from our controls; and third, they provided a comparison group in which follow up biomarkers could be obtained after an ablation procedure. Importantly, as these patients had paroxysmal SVT, they were in sinus rhythm the great majority of the time.
A previous study demonstrated that IL-6 and tumor necrosis factor-α were elevated in the coronary sinus compared to the aortic root in 36 patients with acute coronary syndromes. Given that CRP and IL-6 are expressed in myocardium due to a variety of stressors,21,22 we hypothesized that the elevation in inflammatory markers seen in the peripheral venous system would originate in the heart and therefore be higher in the coronary sinus. Interestingly, whereas levels of both markers were similar in the femoral vein and the coronary sinus in the control patients, they were both significantly lower in the coronary sinus of the AFL patients. As with the other significant differences observed in this study, these lower coronary sinus levels were driven by the patients presenting in AFL or AF. This suggests that the peripheral elevation seen is due to some systemic process rather than one unique to the heart. Another potential explanation is that the inflammatory markers are more active and therefore absorbed to a greater degree in the myocardial cells than in peripheral cells (particularly as cytokines may undergo endocytosis), leading to lower levels in the coronary sinus (distal to myocardial tissue). This may be further investigated by measuring inflammatory markers in multiple compartments, such as the femoral vein, femoral artery, left atrium and coronary sinus. Finally, we cannot exclude the possibility that some other agent of inflammation is present in greater concentrations in the cardiac circulation and that CRP and IL-6 are epiphenomena, released as part of a more general systemic response.
The mechanism by which AFL might contribute to inflammation requires further elucidation. CRP has been shown to be elevated with higher left ventricular end-diastolic pressure;23 therefore our findings may simply reflect hemodynamic changes. Specifically, reversible atrial and/or ventricular mechanical dysfunction due to persistently elevated rates may be the primary cause of the elevated inflammatory markers. Important directions for future research might include investigating the relationship between inflammatory markers and dynamic changes in atrial and ventricular function, either by echocardiogram or magnetic resonance imaging.
This study has several limitations. First, not all patients had coronary sinus blood available nor did all patients return for follow up. However, in both instances, neither baseline clinical characteristics nor baseline femoral biomarker levels were different in those with and without missing data. Second, although we excluded patients with known or clinically evident inflammatory conditions, we cannot absolutely exclude the possibility that some (AFL) patients had subclinical inflammatory disease at baseline that coincidentally resolved by the time of the first follow up visit. Third, we did not have echocardiograms on all patients and therefore could not thoroughly assess relationships between the inflammatory markers and cardiac chamber size or function.
Conclusion
The presence of AFL or AF, but not necessarily a history of AFL, is associated with evidence of elevated CRP and IL-6. As evidenced by lower levels of these markers in the coronary sinus in those with AFL, this inflammation does not appear to originate in the heart, but rather appears to be a systemic response. Finally, this inflammation resolves with ablation of AFL, suggesting that the elevation of these markers is an effect rather than a cause of the atrial tachyarrhythmia.
Acknowledgments
Funding Sources
This work was made possible by a Heart Rhythm Society Fellowship Award (G.M.M.), Grant Number K12 RR024130 (G.M.M.) from the National Center for Research Resources (NCRR), a component of the NIH and NIH Roadmap for Medical Research, American Heart Association Western States Affiliate Beginning Grant-in-Aid Award (G. M. M.), and the National Heart, Lung, and Blood Institute grant RO1-HL072854 (J.E.O.) and NIH.
Abbreviations
- AF
atrial fibrillation
- AFL
atrial flutter
- SVT
Paroxysmal supraventricular tachycardia
- CS
Coronary sinus
- CRP
C-Reactive Protein
- IL-6
Interleukin 6
- AVNRT
AV nodal reentrant tachycardia
- AVRT
Atrioventricular reciprocating tachycardia
- IQR
Interquartile range
- CI
Confidence interval
- BMI
Body mass index
- EF
Ejection fraction
- ARB
Angiotensin receptor blocker
Footnotes
Disclosures/potential conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–378. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 2.Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, Bauer JA, Tchou PJ, Niebauer MJ, Natale A, Van Wagoner DR. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Zamorano JL. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation) J Am Coll Cardiol. 2006;48:854–906. doi: 10.1016/j.jacc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Waldo AL. The interrelationship between atrial fibrillation and atrial flutter. Prog Cardiovas Disease. 2005;48:41–56. doi: 10.1016/j.pcad.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Calkins H, Leon AR, Deam AG, Kalbfleisch SJ, Langberg JJ, Morady F. Catheter ablation of atrial flutter using radiofrequency energy. Am J Cardiol. 1994;73:353–356. doi: 10.1016/0002-9149(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 6.Saxon LA, Kalman JM, Olgin JE, Scheinman MM, Lee RJ, Lesh MD. Results of radiofrequency catheter ablation for atrial flutter. Am J Cardiol. 1996;77:1014–1016. doi: 10.1016/s0002-9149(97)89162-x. [DOI] [PubMed] [Google Scholar]
- 7.Tai CT, Chen SA, Chiang CE, Tai CT, Chen SA, Chiang CE, Lee SH, Wen ZC, Huang JL, Chen YJ, Yu WC, Feng AN, Lin YJ, Ding YA, Chang MS. Long-term outcome of radiofrequency catheter ablation for typical atrial flutter: risk prediction of recurrent arrhythmias. J Cardiovasc Electrophysiol. 1998;9:115–121. doi: 10.1111/j.1540-8167.1998.tb00892.x. [DOI] [PubMed] [Google Scholar]
- 8.Blomstrom-Lundqvist C, Scheinman MM, Aliot EM, Alpert JS, Calkins H, Camm AJ, Campbell WB, Haines DE, Kuck KH, Lerman BB, Miller DD, Shaeffer CW, Jr, Stevenson WG, Tomaselli GF, Antman EM, Smith SC, Jr, Alpert JS, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Hiratzka LF, Hunt SA, Jacobs AK, Russell RO, Jr, Priori SG, Blanc JJ, Budaj A, Burgos EF, Cowie M, Deckers JW, Garcia MA, Klein WW, Lekakis J, Lindahl B, Mazzotta G, Morais JC, Oto A, Smiseth O, Trappe HJ. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Supraventricular Arrhythmias) Circulation. 2003;108:1871–1909. doi: 10.1161/01.CIR.0000091380.04100.84. [DOI] [PubMed] [Google Scholar]
- 9.Lee KW, Yang Y, Scheinman MM. Atrial flutter: a review of its history, mechanisms, clinical features, and current therapy. Curr Probl Cardiol. 2005;30:121–167. doi: 10.1016/j.cpcardiol.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Conway DS, Buggins P, Hughes E, Lip GY. Prognostic significance of raised plasma levels of interleukin-6 and C-reactive protein in atrial fibrillation. Am Heart J. 2004;148:462–466. doi: 10.1016/j.ahj.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Psychari SN, Apostolou TS, Sinos L, Hamodraka E, Liakos G, Kremastinos DT. Relation of elevated C-reactive protein and interleukin-6 levels to left atrial size and duration of episodes in patients with atrial fibrillation. Am J Cardiol. 2005;95:764–767. doi: 10.1016/j.amjcard.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 13.Kumagai K, Khrestian C, Waldo AL. Simultaneous multisite mapping studies during induced atrial fibrillation in the sterile pericarditis model. Insights into the mechanism of its maintenance. Circulation. 1997;95:511–521. doi: 10.1161/01.cir.95.2.511. [DOI] [PubMed] [Google Scholar]
- 14.Bruins P, te Velthuis H, Yazdanbakhsh AP, Jansen PG, van Hardevelt FW, de Beaumont EM, Wildevuur CR, Eijsman L, Trouwborst A, Hack CE. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation. 1997;96:3542–3548. doi: 10.1161/01.cir.96.10.3542. [DOI] [PubMed] [Google Scholar]
- 15.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 16.Sata N, Hamada N, Horinouchi T, Amitani S, Yamashita T, Moriyama Y, Miyahara K. C-reactive protein and atrial fibrillation. Is inflammation a consequence or a cause of atrial fibrillation? Jpn Heart J. 2004;45:441–445. doi: 10.1536/jhj.45.441. [DOI] [PubMed] [Google Scholar]
- 17.Buob A, Jung J, Siaplaouras S, Neuberger HR, Mewis C. Discordant regulation of CRP and NT-proBNP plasma levels after electrical cardioversion of persistent atrial fibrillation. Pacing Clin Electrophysiol. 2006;29:559–563. doi: 10.1111/j.1540-8159.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 18.Marcus GM, Sung RJ. Antiarrhythmic agents in facilitating electrical cardioversion of atrial fibrillation and promoting maintenance of sinus rhythm. Cardiology. 2001;95:1–8. doi: 10.1159/000047335. [DOI] [PubMed] [Google Scholar]
- 19.Page RL, Wilkinson WE, Clair WK, McCarthy EA, Pritchett EL. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89:224–227. doi: 10.1161/01.cir.89.1.224. [DOI] [PubMed] [Google Scholar]
- 20.Morady F. Catheter ablation of supraventricular arrhythmias: state of the art. J Cardiovasc Electrophysiol. 2004;15:124–139. doi: 10.1046/j.1540-8167.2004.03516.x. [DOI] [PubMed] [Google Scholar]
- 21.Satoh M, Nakamura M, Akatsu T, Shimoda Y, Segawa I, Hiramori K. C-reactive protein co-expresses with tumor necrosis factor-alpha in the myocardium in human dilated cardiomyopathy. Eur J Heart Fail. 2005;7:748–754. doi: 10.1016/j.ejheart.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 22.Palmieri EA, Benincasa G, Di Rella F, Casaburi C, Monti MG, De Simone G, Chiariotti L, Palombini L, Bruni CB, Sacca L, Cittadini A. Differential expression of TNF-alpha, IL-6, and IGF-1 by graded mechanical stress in normal rat myocardium. Am J Physiol Heart Circ Physiol. 2002;282:H926–934. doi: 10.1152/ajpheart.00436.2001. [DOI] [PubMed] [Google Scholar]
- 23.Shah SJ, Marcus GM, Gerber IL, McKeown BH, Vessey JC, Jordan MV, Huddleston M, Foster E, Chatterjee K, Michaels AD. High-sensitivity C-reactive protein and parameters of left ventricular dysfunction. J Card Fail. 2006;12:61–65. doi: 10.1016/j.cardfail.2005.08.003. [DOI] [PubMed] [Google Scholar]