Abstract
The antiphospholipid syndrome (APS) is a leading cause of miscarriage and maternal and fetal morbidity. APS is characterized by thrombosis and pregnancy loss that occur in the presence of antiphospholipid (aPL) antibodies. Using a mouse model of APS induced by passive transfer of human aPL antibodies, we have shown that complement activation plays an essential and causative role in pregnancy loss and fetal growth restriction, and that blocking activation of the complement cascade rescues pregnancies. Conventional treatment for APS patients is sub-anticoagulant doses of heparin throughout pregnancy. Could heparin prevent pregnancy loss by inhibiting complement? In our experimental model of APS, heparin inhibits activation of complement on trophoblasts in vivo and in vitro, and anticoagulation in and of itself is not sufficient to prevent pregnancy complications. These studies underscore the importance of inflammation in fetal injury associated with aPL antibodies and raise the importance of developing and testing targeted complement inhibitory therapy for patients with APS.
Introduction
The antiphospholipid antibody syndrome (APS) is characterized by arterial and venous thrombosis and pregnancy complications in association with antiphospholipid (aPL) antibodies. In addition to recurrent miscarriage and fetal death, pregnancy complications in women with APS include preeclampsia, placental insufficiency, and fetal growth restriction (Wilson et al., 1999; Lockshin et al., 2000; Levine et al., 2002). The pathogenic mechanisms that lead to injury in vivo are incompletely understood and therapy for pregnant women with APS, currently aimed at preventing thrombosis (Levine et al., 2002; Derksen et al., 2004), is only partially successful in averting pregnancy loss.
Recent experimental observations suggest that altered regulation of complement, an ancient component of the innate immune system, can cause and may perpetuate complications of pregnancy (Xu et al., 2000; Girardi et al., 2003). We have found that aPL antibodies mediate pregnancy complications by initiating activation of the complement cascade, and that the local increase in complement activation fragments is highly deleterious to the developing fetus (Holers et al., 2002a; Girardi et al., 2003). Thus, the identification of this new mechanism for pregnancy loss in women with aPL antibodies holds the promise of new, safer and better treatments.
Complement activation, tissue injury and fetal tolerance
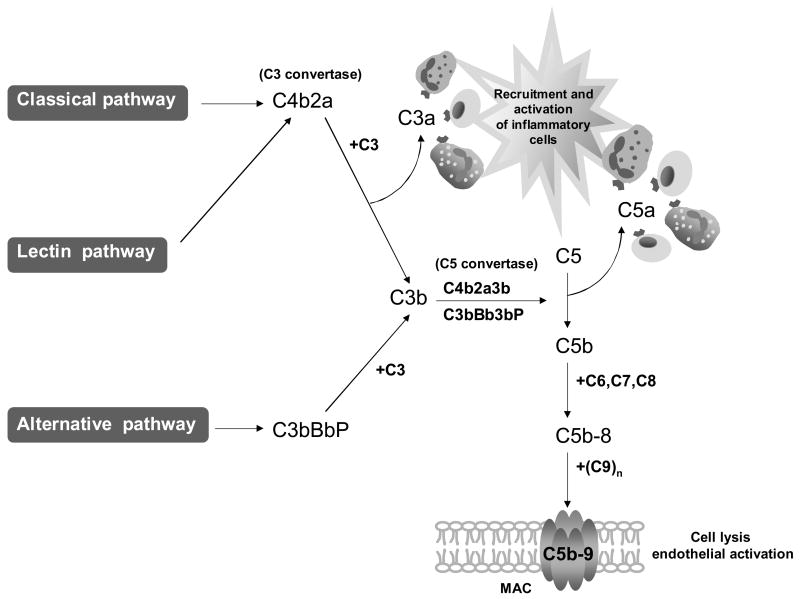
The complement system, composed of over 30 proteins that act in concert to protect the host against invading organisms, initiates inflammation and tissue injury (Figure 1) (Abbas et al., 2000; Schmidt and Colten, 2000). Complement activation promotes recruitment and activation of inflammatory cells. The classical pathway is activated when antibodies bind to antigen and unleash potent effectors associated with humoral responses in immune-mediated tissue damage. The mannose-binding lectin (MBL) pathway is activated by MBL recognition of carbohydrates (often on infectious agents). Alternative pathway activation mechanisms differ in that they are initiated by the binding of spontaneously activated complement components to the surface of pathogens. Recent data show that oxidative stress initiates complement activation by all three pathways (Thurman et al., 2003; Hart et al., 2004; Gadjeva et al., 2004). By means of these recognition and activation mechanisms, the complement system identifies and responds to ‘dangerous’ situations presented by foreign antigens, pathogens, tissue injury, ischemia, apoptosis and necrosis (Fearon, 1997). This capacity places the complement system at the center of many clinically important responses to pathogens, as well as to fetal injury mediated by cellular or humoral immune mechanisms.
Figure 1.
Complement cascade. Schematic diagram of the three complement activation pathways and the products they generate. From Hughes Syndrome, 2nd Edition, Khamashta, MA (Ed.), 2006, page 396, chapter 31, by Girardi, G and Salmon, J, Figure 31.1. With kind permission of Springer Science and Business Media.
The convergence of three complement activation pathways on the C3 protein results in a common pathway of effector functions (Figure 1). The initial step is generation of the fragments C3a and C3b. C3a, an anaphylatoxin that binds to receptors on leukocytes and other cells, causes activation and release of inflammatory mediators (Hugli, 1990). C3b and its further sequential cleavage fragments are ligands for complement receptors 1 and 2 and the β2 integrins, CD11b/CD18 and CD11c/CD18, present on a variety of inflammatory and immune accessory cells (Brown, 1991; Holers, 1995). C3b attaches covalently to targets, followed by the assembly of C5 convertase with subsequent cleavage of C5 to C5a and C5b. C5a is a potent soluble inflammatory, anaphylatoxic and chemotactic molecule that promotes recruitment and activation of neutrophils and monocytes and mediates endothelial cell activation through its receptor. Binding of C5b to the target initiates the non-enzymatic assembly of the C5b-9 membrane attack complex (MAC). Insertion of C5b-9 MAC into cell membranes causes erythrocyte lysis through changes in intracellular osmolarity, while C5b-9 MAC damages nucleated cells primarily by activating specific pro-inflammatory signaling pathways (Rus et al., 2001; Morgan and Meri, 1994).
Because activated complement fragments have the capacity to bind and damage self-tissues, it is imperative that autologous bystander cells be protected from the deleterious effects of complement. To this end, most human and murine cells express soluble and membrane-bound molecules that limit the activation of various complement components (Abbas et al., 2000; Song, 2004). Though activated complement components are present in normal placentas (Weir, 1981; Wells et al., 1987), it appears that uncontrolled uncontrolled complement activation is prevented in successful pregnancy by three regulatory proteins present on the trophoblast membrane: decay accelerating factor (DAF), membrane cofactor protein (MCP) and CD59 (Cunningham and Tichenor, 1995; Tedesco et al., 1993; Liszewski et al., 1996). All three proteins are strategically positioned on the trophoblast and provide a mechanism to protect the fetus from damage due to complement pathway activation.
That appropriate complement inhibition is an absolute requirement for normal pregnancy has been demonstrated by the finding that deficiency of Crry (a membrane-bound complement regulatory protein, like DAF and MCP, that blocks C3 and C4 activation) leads to progressive embryonic lethality in mice (Xu et al., 2000). Importantly, Crry−/− embryos are rescued when C3 deficiency or factor B deficiency is introduced to Crry−/− embryos (Xu et al., 2000; Mao et al., 2002). Based on the evidence that Crry−/− embryos die due to their inability to suppress complement activation mediated by C3, we proposed that aPL antibodies activate complement within decidual tissue, overwhelm the normally adequate inhibitory mechanisms and induce inflammation and fetal damage.
Role of complement in fetal damage induced by aPL antibodies in a mouse model
During trophoblast differentiation, phosphatidylserine is externalized on the trophoblast outer leaflet where it provides a target for aPL antibodies (Rote and Stetzer, 2003; Rote et al., 1998). We hypothesized that aPL antibodies bound to trophoblasts activate complement via the classical pathway generating split products that mediate placental injury and cause fetal loss and growth restriction. Using a murine model of APS induced by passive transfer of human aPL antibodies, we have shown that complement activation plays an essential and causative role in pregnancy loss and fetal growth restriction (Girardi et al., 2003; Holers et al., 2002b). Passive transfer of IgG from women with recurrent miscarriage and aPL antibodies results in a 40% frequency of fetal resorption compared to <10% in mice treated with IgG from healthy individuals, and a 35% reduction in the average weight of surviving fetuses (Holers et al., 2002b). We observed a similar rate of pregnancy failure using IgG from 5 different patients as well as monoclonal human aPL antibodies. These in vivo pathogenic effects require both recognition of relevant target antigens by aPL antibodies and Fc domain-mediated complement activation that initiates effector functions.
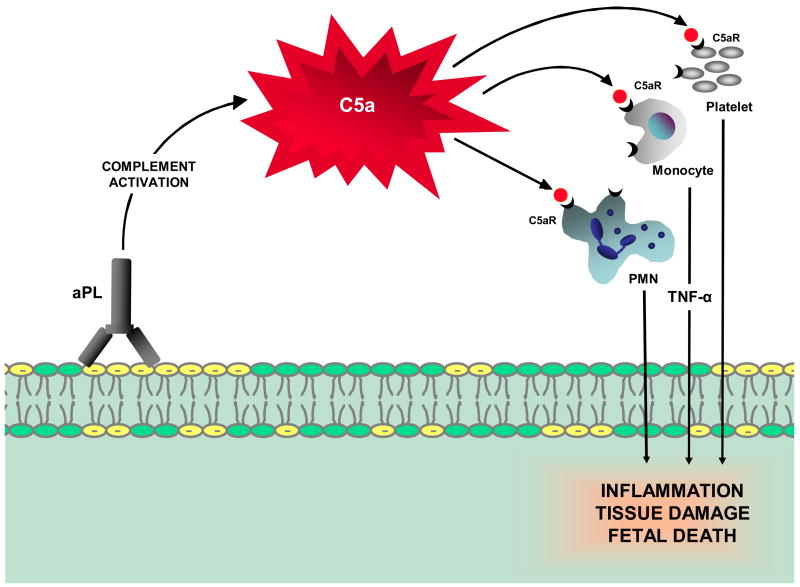
In our initial studies, we found that inhibition of the complement cascade in vivo, using the C3 convertase inhibitor Crry-Ig, prevented fetal loss and growth restriction and that mice deficient in complement C3 were resistant to fetal injury induced by aPL antibodies. To define the initiating pathways and critical effectors of aPL-induced pregnancy injury, we used mice deficient in complement elements (C4, factor B, C5 and C5a receptor) and inhibitors of complement activation (anti-C5 mAb, anti-factor B mAb and C5a receptor antagonist peptide) in our mouse model of APS. We identified complement component C5, and particularly its cleavage product C5a, as key mediators of fetal injury and showed that antibodies or peptides that block C5a-C5a receptor interactions prevent pregnancy complications (Figure 2). Furthermore, our results indicate that both classical and alternative complement pathway activation contribute to damage. Mice deficient in alternative and classical pathway complement components (factor B, C4, C3 and C5) were resistant to fetal injury induced by aPL antibodies.
Figure 2.
Proposed mechanism for the pathogenic effects of aPL antibodies on tissue injury. APL antibodies are preferentially targeted to the placenta where they activate complement via the classical pathway. The complement cascade is initiated leading to generation of C5a and recruitment and activation of neutrophils, monocytes and platelet cells, and release of inflammatory mediators, including reactive oxidants, proteolytic enzymes, cytokines, chemokines and complement factors. Depending on the extent of damage, either death in utero or fetal growth restriction ensues.
We have begun studies to define the downstream effectors of complement activation. We found that aPL antibodies, specifically targeted to decidual tissue, cause a rapid increase in decidual and systemic TNF-α levels. The release of TNF-α is a critical intermediate that acts downstream of C5 activation. In C5-deficient mice treated with aPL antibodies, there is no increase in TNF-α levels and, in mice deficient in TNF-α or treated with TNF-α blockade, fetal resorption is less frequent (Berman et al., 2005). Our results suggest that TNF-α is one mediator that links complement activation and pathogenic aPL antibodies to fetal damage.
Based on the results of our mouse studies, we proposed a mechanism for pregnancy complications associated with aPL antibodies: aPL antibodies preferentially targeted at decidua and placenta activate complement via the classical pathway (Fc- and C4-dependent), leading to the generation of potent anaphylatoxins (C3a and C5a) and mediators of effector cell activation, including TNF-α. Recruitment of inflammatory cells accelerates local alternative pathway activation and creates a proinflammatory amplification loop that enhances C3 activation and deposition, generates additional C3a and C5a, and results in further influx of inflammatory cells into the placenta. Depending on the extent of damage, either death in utero or fetal growth restriction ensues.
Heparin prevents aPL antibody-induced fetal loss by inhibiting complement activation
These studies underscore the importance of inflammation, rather than thrombosis, in fetal injury associated with aPL antibodies, yet therapy for pregnant women with APS is focused on preventing thrombosis and anticoagulation is only partially successful in averting miscarriage. Histopathologic findings in placentas from women with APS also argue that pro-inflammatory factors may contribute to tissue injury (Out et al., 1991; Magid et al., 1998). Given that the primary treatment for APS patients is anticoagulation throughout pregnancy, usually with sub-anticoagulant doses of heparin, and evidence that heparin inhibits complement activation in vitro, we considered the possibility that heparin prevents pregnancy loss by inhibiting complement activation on trophoblasts and that anticoagulation, in and of itself, is not sufficient to prevent pregnancy complications in APS. We found that treatment with unfractionated heparin or low molecular weight heparin protected pregnancies from aPL-induced damage even at doses that did not cause detectable interference with coagulation. In contrast, treatment with hirudin or fondaparinux (anticoagulants without anti-complement effects) was not protective demonstrating that anticoagulation is insufficient therapy for APS-associated miscarriage (Girardi et al., 2004). Furthermore, heparins inhibited both aPL antibody-induced elevations in circulating C3a and increased C3b deposition in decidual tissues (neither was altered by the other anticoagulants) and blocked C3 cleavage in vitro. Thus, heparin may prevent pregnancy complications by limiting complement activation and the ensuing inflammatory response at the maternal-fetal interface, rather than by inhibiting thrombosis. This work provides a framework for understanding how sub-anticoagulant doses of heparin exert beneficial effects in antibody-mediated tissue injury.
Conclusions
Our experiments indicate that aPL antibodies targeted to decidual tissues damage pregnancies by engagement of the classical pathway of complement activation, followed by amplification through the alternative pathway, and it appears the heparins prevent obstetrical complications caused by aPL antibodies because they block activation of complement. We have initiated the the PROMISSE Study (Predictors of pRegnancy Outcome: bioMarkers In antiphospholipid antibody Syndrome and Systemic lupus Erythematosus), a prospective, multi-center observational study to translate our findings in mice to humans and evaluate the role of complement in aPL antibody-induced pregnancy loss in women. The PROMISSE Study will test the hypothesis that classical, alternative and terminal complement pathway activation will be detected in the circulation and placentas of patients with aPL antibodies and will be associated with poor pregnancy outcomes. Characterization of biomarkers that predict poor pregnancy outcome will identify clinically applicable indicators that will enable us to initiate an interventional trial, perhaps using complement inhibitors, in patients at risk for aPL antibody-associated fetal loss.
Acknowledgments
This research was supported in part by grants from NIH, the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery, the Alliance for Lupus Research, and the S.L.E. Foundation, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas AK, Lichtman AH, Pober JS. Cellular and Molecular Immunology. W.B. Saunders Company; Philadelphia, PA: 2000. pp. 316–334. [Google Scholar]
- Berman J, Girardi G, Salmon JE. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J Immunol. 2005;174:485–490. doi: 10.4049/jimmunol.174.1.485. [DOI] [PubMed] [Google Scholar]
- Brown EJ. Complement receptors and phagocytosis. Curr Opin Immunol. 1991;3:76–82. doi: 10.1016/0952-7915(91)90081-b. [DOI] [PubMed] [Google Scholar]
- Cunningham DS, Tichenor JR., Jr Decay-accelerating factor protects human trophoblast from complement-mediated attack. Clin Immunol Immunopathol. 1995;74:156–161. doi: 10.1006/clin.1995.1023. [DOI] [PubMed] [Google Scholar]
- Derksen RH, Khamashta MA, Branch DW. Management of the obstetric antiphospholipid syndrome. Arthr Rheum. 2004;50:1028–1039. doi: 10.1002/art.20105. [DOI] [PubMed] [Google Scholar]
- Fearon DT. Seeking wisdom in innate immunity. Nature. 1997;388:323–324. doi: 10.1038/40967. [DOI] [PubMed] [Google Scholar]
- Gadjeva M, Takahashi K, Thiel S. Mannan-binding lectin--a soluble pattern recognition molecule. Mol Immunol. 2004;41:113–121. doi: 10.1016/j.molimm.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA, Lambris JD, Holers VM, Salmon JE. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644–54. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10:1222–1226. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- Hart ML, Walsh MC, Stahl GL. Initiation of complement activation following oxidative stress. In vitro and in vivo observations. Mol Immunol. 2004;41:165–171. doi: 10.1016/j.molimm.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Holers VM. In: Complement; principles and practices of clinical immunology. Rich R, editor. Mosby; St. Louis, MO: 1995. p. 363. [Google Scholar]
- Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, Espinola R, Xiaowei LE, Mao D, Vialpando CG, Salmon JE. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002a;195:211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, Espinola R, Xiaowei LE, Mao D, Vialpando CG, Salmon JE. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002b;195:211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugli TE. Structure and function of C3a anaphylatoxin. Curr Top Microbiol Immunol. 1990;153:181–208. doi: 10.1007/978-3-642-74977-3_10. [DOI] [PubMed] [Google Scholar]
- Levine JS, Branch DW, Rauch J. The antiphospholipid syndrome. N Engl J Med. 2002;346:752–763. doi: 10.1056/NEJMra002974. [DOI] [PubMed] [Google Scholar]
- Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Adv Immunol. 1996;61:201–283. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- Lockshin MD, Sammaritano LR, Schwartzman S. Validation of the Sapporo criteria for antiphospholipid syndrome. Arthr Rheum. 2000;43:440–443. doi: 10.1002/1529-0131(200002)43:2<440::AID-ANR26>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Magid MS, Kaplan C, Sammaritano LR, Peterson M, Druzin ML, Lockshin MD. Placental pathology in systemic lupus erythematosus: a prospective study. Am J Obstet Gynecol. 1998;179:226–234. doi: 10.1016/s0002-9378(98)70277-7. [DOI] [PubMed] [Google Scholar]
- Mao D, Wu X, Deppong C, Friend LD, Dolecki G, Nelson DM, Molina H. Negligible role of antibodies and C5 in pregnancy loss associated exclusively with C3-dependent mechanisms through complement alternative pathway. Immunity. 2003;19:813–822. doi: 10.1016/s1074-7613(03)00321-2. [DOI] [PubMed] [Google Scholar]
- Morgan BP, Meri S. Membrane proteins that protect against complement lysis. Springer Semin Immunopathol. 1994;15:369–396. doi: 10.1007/BF01837366. [DOI] [PubMed] [Google Scholar]
- Out HJ, Kooijman CD, Bruinse HW, Derksen RH. Histopathological findings in placentae from patients with intra-uterine fetal death and anti-phospholipid antibodies. Eur J Obstet Gynecol Reprod Biol. 1991;41:179–186. doi: 10.1016/0028-2243(91)90021-c. [DOI] [PubMed] [Google Scholar]
- Rote NS, Stetzer BP. Autoimmune disease as a cause of reproductive failure. Clin Lab Med. 2003;23:265–293. doi: 10.1016/s0272-2712(03)00022-2. [DOI] [PubMed] [Google Scholar]
- Rote NS, Vogt E, DeVere G, Obringer AR, Ng AK. The role of placental trophoblast in the pathophysiology of the antiphospholipid antibody syndrome. Am J Reprod Immunol. 1998;39:125–136. doi: 10.1111/j.1600-0897.1998.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Rus HG, Niculescu FI, Shin ML. Role of the C5b-9 complement complex in cell cycle and apoptosis. Immunol Rev. 2001;180:49–55. doi: 10.1034/j.1600-065x.2001.1800104.x. [DOI] [PubMed] [Google Scholar]
- Schmidt BZ, Colten HR. Complement: a critical test of its biological importance. Immunol Rev. 2000;178:166–176. doi: 10.1034/j.1600-065x.2000.17801.x. [DOI] [PubMed] [Google Scholar]
- Song WC. Membrane complement regulatory proteins in autoimmune and inflammatory tissue injury. Curr Dir Autoimmun. 2004;7:181–199. doi: 10.1159/000075693. [DOI] [PubMed] [Google Scholar]
- Tedesco F, Narchi G, Radillo O, Meri S, Ferrone S, Betterle C. Susceptibility of human trophoblast to killing by human complement and the role of the complement regulatory proteins. J Immunol. 1993;151:1562–1570. [PubMed] [Google Scholar]
- Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM. Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol. 2003;170:1517–1523. doi: 10.4049/jimmunol.170.3.1517. [DOI] [PubMed] [Google Scholar]
- Weir PE. Immunofluorescent studies of the uteroplacental arteries in normal pregnancy. Br J Obstet Gynaecol. 1981;88:301–317. doi: 10.1111/j.1471-0528.1981.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Wells M, Bennett J, Bulmer JN, Jackson P, Holgate CS. Complement component deposition in uteroplacental (spiral) arteries in normal human pregnancy. J Reprod Immunol. 1987;12:125–135. doi: 10.1016/0165-0378(87)90040-4. [DOI] [PubMed] [Google Scholar]
- Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, Brey R, Derksen R, Harris EN, Hughes GR, Triplett DA, Khamashta MA. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthr Rheum. 1999;42:1309–1311. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000;287:498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]