Abstract
Until very recently, comparatively few scientists were studying hallucinogenic drugs. Nevertheless, selective antagonists are available for relevant serotonergic receptors, the majority of which have now been cloned, allowing for reasonably thorough pharmacological investigation. Animal models sensitive to the behavioral effects of the hallucinogens have been established and exploited. Sophisticated genetic techniques have enabled the development of mutant mice, which have proven useful in the study of hallucinogens. The capacity to study post-receptor signaling events has lead to the proposal of a plausible mechanism of action for these compounds. The tools currently available to study the hallucinogens are thus more plentiful and scientifically advanced than were those accessible to earlier researchers studying the opioids, benzodiazepines, cholinergics, or other centrally active compounds. The behavioral pharmacology of phenethylamine, tryptamine, and ergoline hallucinogens are described in this review, paying particular attention to important structure activity relationships which have emerged, receptors involved in their various actions, effects on conditioned and unconditioned behaviors, and in some cases, human psychopharmacology. As clinical interest in the therapeutic potential of these compounds is once again beginning to emerge, it is important to recognize the wealth of data derived from controlled preclinical studies on these compounds.
Introduction
Although drugs producing sensory distortions have been used by man for several millennia, many consider the modern era of psychedelics to have begun when the psychotropic effects of lysergic acid diethylamide (LSD, Figure 1, C.) were discovered by Albert Hofmann in 1943 [1]. This discovery ushered in an era of intense LSD research, with nearly 1,000 articles appearing in the medical literature by 1961 [2]. Most of this early research was based upon the drug's capacity to produce a “model psychosis” [3] although there are significant differences between LSD-induced and endogenously occurring psychotic behaviors [4]. By the mid 1960s, LSD and other related drugs had become associated with various counterculture movements, depicted as dangerous, and widely popularized as drugs of abuse. Accordingly, scientific interest in these drugs faded by the late 1960s, but human research with related psychedelics has recently experienced a slight renaissance [5 - 13].
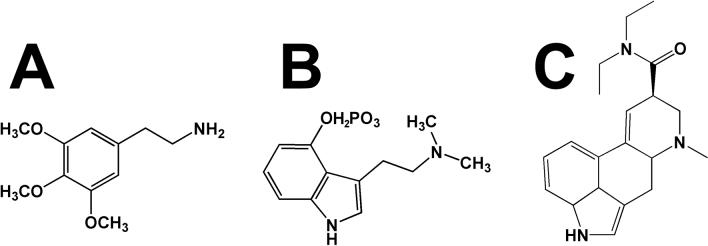
Figure 1.
Chemical structures of the prototypical phenethylamine hallucinongen 3,4,5-trimethoxy-phenethylamine (mescaline, A.), the representative tryptamine hallucinogen N,N-dimethyl-4-phosphoryloxytryptamine (psilocybin, B.), and the archetypal ergoline hallucinogen lysergic acid diethylamide (LSD, C.).
The term “hallucinogen” has come to describe LSD and related compounds based on the supposition that these drugs elicit hallucinations, but it has been argued that, at the doses commonly taken recreationally, frank hallucinations are produced only rarely [14]. Nevertheless, other designations for this class of drugs (for example, psychedelics, psychotomimetics, entheogens, etc.) have not necessarily caught on, and so we will use the term hallucinogen to refer to these compounds, despite the controversy surrounding the appropriateness of this appellation. As a drug category, hallucinogens are typically accepted to encompass an enormous range of pharmacological substances, with mechanisms of action ranging from cannabinoid agonism (i.e., Δ9-tetrahydrocannabinol), N-methyl-D-aspartate (NMDA) antagonism (i.e., phencyclidine), muscarinic receptor antagonism (i.e., scopolamine), κ opioid agonism (i.e., salvinorin A), mixed action monoamine release (i.e., 3,4-methylenedioxymethamphetamine [MDMA]), and more. Thus, within the confines of this review, we will use the term hallucinogen to denote compounds with pharmacological effects similar to three prototypical drugs: 3,4,5-trimethoxy-phenethylamine (mescaline, Figure 1, A.), N,N-dimethyl-4-phosphoryloxytryptamine (psilocybin, Figure 1, B.) and LSD (Figure 1, C.). All of these drugs function as agonists at 5-HT2A receptors, and much work has culminated in the widespread acceptance that this particular receptor initiates the molecular mechanisms responsible for the unique effects of these compounds. Much of that work will be reviewed herein.
The aim of this review is to mark the sea change which seems to be occurring within the field of hallucinogen research. Until very recently, comparatively few scientists were studying these particular compounds, perhaps due to their unfortunate association with somewhat less than rigorous research techniques. In Nichols' recent review [14], for example, prominent clinicians are quoted as stating that the effects of hallucinogens transcend pharmacology, are unpredictable, and border on the mystical. Nevertheless, the state of hallucinogen research is now approaching something of a high water mark. Selective antagonists are available for relevant serotonergic receptors, the majority of which have now been cloned, allowing for reasonably thorough pharmacological investigation. Animal models sensitive to hallucinogen-like effects have been established and exploited to yield a wealth of largely concordant data. Along similar lines, sophisticated genetic techniques have enabled the development of mutant mice, which have proven useful in the study of hallucinogens. Finally, the capacity to study post-receptor signaling events has lead to the proposal of a plausible mechanism of action for these compounds. The tools currently available to study the hallucinogens are thus more plentiful and scientifically advanced than were those accessible to earlier researchers studying the opioids, benzodiazepines, cholinergics, or other centrally active compounds. Those interested in hallucinogen research should thus be encouraged by all of these recent developments, and it is hoped that the perceived “scientific respectability” of the field will continue to increase.
Animal models of hallucinogen-like action
Drug discrimination
Given the profound effects of hallucinogens on perception and other subjective variables, an animal model capable of assessing mechanisms of action of these drugs that informs their subjective effects in man would be especially useful. The main methodology presently employed in this regard is drug discrimination. In a typical discrimination task, an animal is trained to emit one response during experimental sessions initiated by the administration of a particular drug (the “training drug”), and a different response during sessions that follow administration of the drug vehicle. During the development of this assay, a diverse array of responses were typically engendered [15], but the majority of such research now involves discriminative responding on one of two operant devices (levers, nose-poke apertures, etc.) maintained by either appetitive reinforcement or shock avoidance. Under these contingencies, the injection condition (training drug versus saline, for example) serves as an interoceptive discriminative stimulus to cue the animal as to which response will be reinforced during a given session, in much the same way as the operant behavior of an animal may be guided by exteroceptive stimuli, such as colored lights or tones of certain frequencies. The drug discrimination assay is thus essentially a drug detection procedure whereby animals are trained to recognize the stimulus effects of a given dose of a particular training drug. During tests, well-trained animals may be administered different doses of the same training drug, or different doses of a novel compound suspected to have similar subjective effects to the training drug. Results of such tests generate asymptotic dose-effect curves where administration of a test compound with stimulus properties similar to those of the training drug dose-dependently increases responding on the drug lever. Similarly, prior treatment with a competitive antagonist tends to produce parallel rightward shifts in these discrimination dose-effect curves. A secondary measure of this assay is response rate. This measure is critical as it provides a built in positive control which ensures that behaviorally active doses of test compounds are reached. As drug dose increases, operant performance is disrupted, thus, if a test drug fails to elicit any drug-appropriate responding up to doses that suppress response rate, it can be safely concluded that the stimulus properties of the test drug do not overlap with those of the training drug. Theoretical data in this regard are presented in Figure 2.
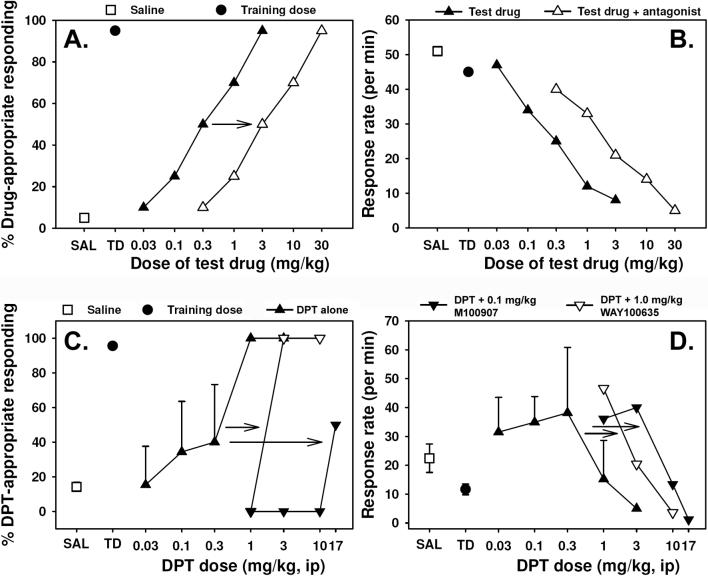
Figure 2.
A. and B. Theoretical drug discrimination data illustrating discriminative control by saline (open square) or the training dose of the training drug (filled circle), dose-dependent substitution of the test drug for the training dose (filled triangles), and a parallel rightward shift in this dose-effect curve produced by prior treatment with a competitive antagonist (open triangles). The low percentage of drug-appropriate responding elicited by saline injection (A.), coupled with the high response rate (B.), demonstrates that the subjects are selecting the saline lever, while the near exclusive high rate responding on the drug lever following injection of the training dose indicates that the subjects have come under stimulus control. Note that increasing doses of the test drug asymptotically increase drug-appropriate responding while decreasing overall response rate. In this illustrative data, antagonist pretreatment attenuates both the discriminative stimulus and rate-decreasing effects of the test drug, but a dissociation of these two effects can be observed under some circumstances. C. and D. Empirical drug discrimination data from mice trained to discriminate 3.0 mg/kg DPT (filled circle) from saline (open square). Panel C. illustrates dose-dependent substitution of DPT for its training dose (filled triangles), and parallel rightward shifts in this dose-effect curve produced by prior treatment with the selective 5-HT1A antagonist WAY100635 or the selective 5-HT2A antagonist M100907 (open triangles). Higher doses of WAY100635 produced no further shift in the discrimination curve and reduced response rates, suggesting that while some component of the discriminative cue induced by DPT is 5-HT1A-mediated, the more salient component is attributable to 5-HT2A receptor stimulation. Panel D. shows that both antagonists reduced the rate-decreasing effects of DPT. These data previously unpublished (Murnane and Fantegrossi).
Complete characterization of a novel compound is no more possible with drug discrimination that it would be with any other single pharmacological procedure, but the strength of the drug discrimination assay lies in its capacity to gauge a range of pharmacological variables relating to the stimulus properties of a drug, including those related to time course (speed of onset, duration of action, etc.), receptor mechanisms important to pharmacological action, similarity of subjective effects to other drugs, structure-activity relationships for a group of chemically-related compounds, and identification and development of antagonists. Another advantage of this procedure is its high degree of pharmacologic specificity. In this regard, well-trained animal will respond on the drug lever when injected with a novel drug that has actions in common with the training drug, in which case the test compound is said to “substitute” for the training drug. Importantly, novel compounds that are active, but pharmacologically dissimilar to the training drug, typically occasion responding on the lever reinforced following administration of the drug vehicle during training sessions. The discriminative stimulus effects of various hallucinogens will be described below.
Drug-elicited head twitch behavior
The drug-elicited head twitch response (HTR) [16 – 17] is a selective behavioral model for 5-HT2A agonist activity in the rodent. The topography of this behavior is similar to a “wet dog shake” and is operationally defined as a rapid, rotational jerk of the head which can be distinguished from species-appropriate grooming or scratching behaviors. Several previous studies have established that direct and indirect 5-HT agonists induce this effect [18 – 25], and that 5-HT2 receptor antagonists selectively block the HTR [26 – 29]. Importantly, the potency with which antagonists attenuate the HTR is highly correlated with the antagonist's affinity for 5-HT2A receptors [18, 30].
The observation of drug-elicited behaviors can be immensely helpful in the initial characterization of the pharmacological actions of new compounds in vivo. For instance, the Straub tail reaction in rodents (contraction of the sacrococcygeus muscle, resulting in erection of the tail) is readily observed following administration of μ opioids, and it has been suggested that observation of this behavior is a sufficient determinant of opioid activity in mice [31]. However, the effects most characteristic of the hallucinogens (such as distortions, intensifications and mixing of the senses, alterations in the perception of the passage of time, etc.) are largely unobservable, even in the human. Nevertheless, the induction of a head twitch response in rodents seems to be a common property of the hallucinogenic drugs, and there is now general agreement that this behavior is mediated by 5-HT2A receptors [32 – 33]. This is not to suggest that all drugs inducing the head twitch response are hallucinogenic. Indeed, head twitches are induced by various serotonergic, but not necessarily hallucinogenic, agents [20, 34]. Thus, the induction of a head twitch response should not be taken as prima facie evidence for hallucinogenic effects without further study. Nevertheless, the establishment of this assay requires little more than a video camera and a steady eye, which may be attractive to researchers wishing to get involved with hallucinogen research. Results of such tests generate biphasic dose-effect curves where administration of an active test compound dose-dependently increases HTR up to some peak amount, and then decreases twitch behavior at higher doses. Prior administration of an antagonist will produce a parallel rightward shift in thee dose effect curves. Theoretical data in this regard are presented in Figure 3. Given the biphasic nature of HTR dose-effect curves, single dose experiments involving the HTR are not particularly informative, particularly when pharmacological challenges are administered and shown to attenuate twitch behavior. In the absence of a full dose-effect curve determination, such attenuation could be due to either true antagonism (the test dose mimics the effects of a lower dose in the presence of the antagonist) or could be due to potentiation (the test dose mimics the effects of a higher dose, which is incompatible with theories of antagonism). The effects of chemically diverse hallucinogens on head twitch behavior will be discussed below.
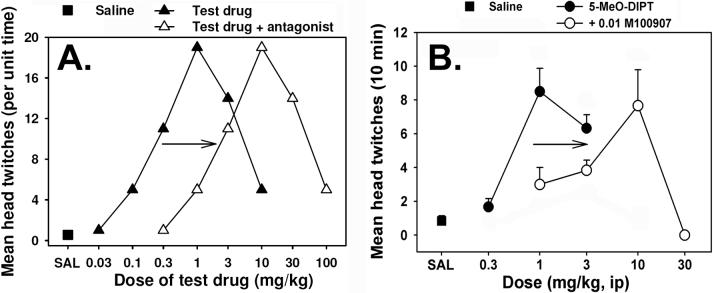
Figure 3.
A. Theoretical head twitch data illustrating low levels of this behavior following injection of saline (filled square), dose-dependent and biphasic effects of a test drug on this behavior (filled triangles), and a parallel rightward shift in this dose-effect curve produced by prior treatment with an antagonist (open triangles). Note that pretreatment with either an additive agonist or an antagonist would decrease the HTR induced by the peak dose of the test drug by functionally increasing or decreasing, respectively, the drug dose. These data emphasize the importance of full dose-effect curve determinations using this assay. B. Empirical head twitch data from mice injected with the tryptamine hallucinogen 5-MeO-DIPT, with or without prior treatment with the selective 5-HT2A antagonist M100907. The antagonist produces a parallel rightward shift in the dose effect curve. Figure replotted and modified slightly from data previously published in [29]; used with permission.
Self-administration
Behaviorism proposes that the actions of an organism are governed by their consequences according to principles of operant conditioning [35 – 36], and the term reinforcement is used to describe the relationship between a behavior and its consequences. Drug self-administration is a technique which allows for the study of drug reinforcement, as the operant response directly produces administration of the pharmacological substance. Such experiments assess the reinforcing effects of a drug by quantifying an increase in the frequency of the behavior which produces drug administration. One critical factor in establishing and maintaining behavior under a given set of contingencies is immediacy of reinforcer delivery, and when studying drugs as reinforcers, this can sometimes pose problems. Essentially, experimenters ask laboratory animals to associate their behavior (for example, the depression of a response lever) with some change in interoceptive state induced by drug administration. Ideally, this change in interoceptive state should occur as rapidly after drug administration as possible in order to facilitate such an association. To the extent that different routes of drug administration can influence onset of drug action, a majority of researchers using drug self-administration procedures have chosen to use intravenous preparations in order to maximize the speed with which drug effects are induced, although it should be noted that delivery of some compounds via intramuscular [37], inhalation [38] and oral [39] routes also maintain behavior. In laboratory animals, intravenous preparations involve the surgical insertion of an indwelling venous catheter (typically into a jugular vein) that is then routed subcutaneously to the mid-scapular region. From here, the catheter either exits the animal and passes through a “tether” system [40 – 41] or connects to a subcutaneously implanted vascular access port [42 – 43]. In either case, the catheter can then be attached to a drug supply via an electronic infusion pump, the operation of which is controlled by the behavior of the animal according to experimenter-determined schedule contingencies.
Despite the reasonably constant recreational use of hallucinogens since at least the early 1970s [44], the reinforcing effects of hallucinogens have not been widely investigated in laboratory animals. Indeed, one of the earliest studies on the reinforcing effects of drugs using the intravenous self-administration procedure in rhesus monkeys found that no animal initiated self-injection of mescaline either spontaneously or after one month of programmed administration [45]. Likewise, the phenethylamine hallucinogen 2,5-dimethoxy-4-methylamphetamine (DOM) was not effective in maintaining self-administration in rhesus monkeys [46]. Nevertheless, the hallucinogen-like phenethylamine 3,4-methylenedioxymethamphetamine (MDMA) has been shown to act as a reinforcer in intravenous self-administration paradigms in baboons [47], rhesus monkeys [48 – 50], rats [51] and mice [52]. Several structural analogues of MDMA, most notably N-ethyl-3,4-methylenedioxyamphetamine [53] and 3,4-methylenedioxyamphetamine [54] (Griffiths et al., 1976), have also been shown to maintain self-administration behavior in baboons.
This is an important point to consider, as the traditional serotonergic hallucinogens of phenethylamine and tryptamine structures have long been distinguished as drugs that are abused by humans but fail to engender reliable self-administration behavior in laboratory animals [55]. These methylenedioxy phenethylamines thus stand in contrast to more traditional hallucinogens such as DOM or mescaline in this regard. Nevertheless, in rhesus monkeys previously trained to self-administer MDMA, several monkeys periodically responded at high rates and earned a majority of all available infusions of mescaline, psilocybin and N,N-dimethyltryptamine (DMT), but did so with no discernible pattern or regularity [56]. This pattern of sporadic self-administration may indicate that these traditional hallucinogens have weak reinforcing effects, or, alternatively, mixed reinforcing and aversive effects. The effects of drug exposure history on initiation of subsequent self-injection and propensity to self-administer specific compounds has been reviewed previously [57], and it seems reasonable to suppose that a behavioral history which includes experience with the reinforcing effects of serotonergic drugs might predispose an organism to self-administer hallucinogenic compounds that have not previously been shown to maintain behavior. A more extensive manipulation of drug history in further studies may greatly improve our understanding of the reinforcing effects of hallucinogens, such as they may be.
Interestingly, hallucinogen-like compounds with antagonist affinity at glutamatergic N-methyl-D-aspartate (NMDA) receptors do maintain self-administration behavior in laboratory animals, as reinforcing effects have been previously demonstrated with phencyclidine (PCP) [58], ketamine [59], and memantine [60]. Furthermore, self-administration of cannabinoid agonists, though slow to be accepted, is now regarded as a robust phenomenon after the convincing demonstrations of the reinforcing effects of Δ9-tetrahydrocannabinol in squirrel monkeys [61 – 62]. These findings may suggest that the appropriate schedules of reinforcement necessary to maintain regular self-administration of phenethylamine and tryptamine hallucinogens have not yet been identified. One of the fundamental tenets of behavioral pharmacology is that the reinforcing effects of drugs are influenced, sometimes profoundly so, by the schedules that govern their contingent delivery [63 – 64]. As data regarding the self-administration of hallucinogens are sparse, there will be little mention of hallucinogen reinforcement for the remainder of this review. However, more work in this regard should be encouraged, as it might ultimately prove helpful in identifying the important behavioral and pharmacological variables which might be relevant to the self-administration of hallucinogens by laboratory animals, and perhaps also to the factors involved in hallucinogen use in humans.
Serotonin receptors and hallucinogen neuropharmacology
Pharmacologists currently recognize seven different serotonin (5-HT) receptors and fourteen different subtypes. The current classification scheme was derived from the explosion of knowledge acquired during the molecular biology revolution of the 1980's and 90's, as newly developed techniques allowed for the determination of sequence homology, leading to a more accurate characterization of new receptor subtypes and the re-classification of some previously-known receptors. Although this work was incredibly important to the study of hallucinogens, the receptor and behavioral pharmacology of such drugs could not be conducted without ligands that specifically bind to those newly distinguished receptors. Only recently has the pharmacology of the serotonin system begun to catch up with the molecular biology. Therefore, the pharmacology of the serotonin system has been largely focused on a subpopulation of the serotonin receptors for which selective ligands have been available.
With the discovery of serotonin as a biologically active substance it was immediately obvious to chemists that LSD and serotonin were structurally similar. However, it was not immediately clear whether LSD mimicked or blocked the effects of serotonin. In fact, initial experiments by John Gaddum and others argued for a mechanism of action wherein LSD blocked the effects of serotonin [65 – 66]. However, this early hypothesis of 5-HT antagonist activity was abandoned when drugs such as 2-bromo-LSD, which function as 5-HT antagonists in peripheral tissues were found to not only lack the subjective effects of LSD [67 – 68] but also to block the subjective effects of LSD itself [69]. These findings, among others, lead to general acceptance that the subjective effects of LSD were in fact mediated through 5-HT receptor agonist activity. The next prominent hypothesis of the mechanism of hallucinogenesis was based on observations by Aghajanian and colleagues that LSD, as well as the simple tryptamines psilocin, DMT, and 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT), suppressed firing of neurons in the dorsal raphe nucleus [70 – 72]. These researchers hypothesized that this mechanism might underlie the hallucinogenic effects of the tryptamines and the ergolines. However, this hypothesis was eventually abandoned because it was found that the putatively non-hallucinogenic ergoline lisuride also suppressed dorsal raphe firing [73], that the phenethylamines lacked this effect entirely [71, 74], and that the suppression of firing lasted longer than the behavioral effects in cat models [75].
More recent work on the mechanisms of hallucinogenesis has focused on the 5-HT2A receptor. This latest hypothesis emerged from work involving drug discrimination procedures which demonstrated that the discriminative stimulus properties of both phenethylamines and tryptamines could be blocked by 5-HT2 receptor antagonists such as ketanserin and pirenperone [76, 19]. This body of work was compelling because it provided a common mechanism of action between the phenethylamines and the tryptamines. Perhaps the most convincing demonstration of 5-HT2-medaited hallucinogen effects was reported in a seminal paper published almost twenty years ago [77]. In these studies, an incredibly tight correlation (r=0.97) between affinity at 5-HT2 receptors and hallucinogenic potency in humans was established. This work provided a persuasive mechanism of action to be more thoroughly explored by future research.
It is important to note, however, that the apparent affinity of agonists for the 5-HT2A receptor estimated from displacement experiments will depend critically on the intrinsic efficacy of the radioligand used to label the binding site. This was elegantly demonstrated by an experiment in which the human 5-HT2A receptor was stably expressed in NIH 3T3 cells, then radiolabeled with [3H]5-HT (a full agonist), [3H] 4-bromo-2,5-dimethoxyamphetamine (DOB, a partial agonist), and [3H]ketanserin (an antagonist) [78]. In this report, receptors labeled with [3H]5-HT displayed a Kd value of 1.3 nM and a Bmax value of 3,461 fmoles/mg protein, and the radiolabeling was sensitive to the stable guanosine 5'-triphosphate (GTP) analogue guanylyl-imidodiphosphate (GMP-PNP). Ketanserin labeled significantly more receptors (Bmax = 27,684 fmoles/mg protein, Kd = 1.1 nM) than did [3H]DOB (Bmax = 8,332 fmoles/mg protein, Kd = 0.8 nM), but both of these agents labeled significantly more receptors than did [3H]5-HT. Most germane to the present argument, agonists had a higher apparent affinity for [3H]-agonist-labeled receptors than for [3H]-antagonist-labeled receptors. Thus, comparisons of affinities for various hallucinogens across multiple sites of action must be regarded as tentative, particularly when distinct receptor populations have been labeled with ligands of varying efficacy.
Glutamatergic and dopaminergic involvement in hallucinogen neuropharmacology
Stimulation of 5-HT2A receptors in brain regions relevant to hallucinogen action is typically associated with an increase in spontaneous glutamate-mediated synaptic activity [79-84]. These findings and others suggest that 5-HT2A receptors may modulate the excitability of specific neural systems, but theories of 5-HT / glutamate (GLU) interactions remain incomplete due the lack of a plausible mechanistic account for this effect. Multiple such mechanistic explanations have been proposed to account for 5-HT2A-mediated stimulation of GLU activity, but the most widely accepted theory implicates an as yet unidentified retrograde messenger capable of triggering GLU release [81, 83, 85]. Although this theory fits a range of data, from cellular to whole animal preparations, the failure thus far to identify the retrograde messenger has precluded rigorous testing. Recently, however, a series of studies used molecular and cellular techniques to directly test different aspects of the retrograde messenger hypothesis, and the resulting findings were inconsistent with this theory [86]. Instead, the authors argued that stimulation of 5-HT2A receptors may lead to an increase in glutamatergic recurrent network activity via direct excitation of specific pyramidal cells in the deeper layers of the prefrontal cortex [86].
Similarly, dopamine D1/D5 receptor agonists may function as neuromodulators that decrease extracellular GLU in prefrontal cortex, an effect which would decrease recurrent network activity induced by hallucinogens [87]. In slices of prefrontal cortex harvested from the rat, it has been demonstrated that low concentrations of D1/D5 agonists suppressed recurrent activity, that their effects were opposite to the enhancement of such activity induced by hallucinogens, and that these dopamine-mediated actions could completely overcome network activity stimulated by 2,5-dimethoxy-4-iodoamphetamine (DOI) [87]. A larger discussion of these effects on neurophysiology is largely outside the scope of this review, but the interested reader is directed to the references cited above for a more thorough treatment of these topics.
Chemical classes of hallucinogens
There are two main chemical classes of hallucinogens, based upon either phenethylamine (mescaline-like) or tryptamine (psilocybin-like) backbones. LSD and a few interesting analogues represent elaborated, conformationally restrained tryptamines, and are commonly referred to as ergolines. Here we dedicate a separate section to LSD and its purportedly non-hallucinogenic analogue lisuride. The behavioral pharmacology of these drugs will be described in the sections below, paying particular attention to important structure activity relationships which have emerged, receptors involved in their behavioral actions, discriminative stimulus effects, capacity to elicit HTR, and in some cases, human psychopharmacology.
Phenethylamines
The chemical backbone for all of the hallucinogenic phenethylamines is based upon the amino acid phenylalanine. Enzymatic decarboxylation biotransforms phenylalanine to phenethylamine, which is a prevalent structure in a range of endogenous compounds, including neurotransmitters and hormones. With regard to drugs of abuse other than hallucinogens, substituted phenethylamines can function as stimulants (i.e., amphetamine), entactogens (i.e., MDMA), and even as opioids (i.e., the morphinans). Although we have grouped LSD with the tryptamines for purposes of this review, it is important to note that the phenethylamine structure can be found as part of the more complex ring system of the ergolines as well. Substituted phenethylamines may be chemically modified along the phenyl ring, the sidechain, or the amino group. In this regard, substituted amphetamines are homologues of phenethylamines containing an α-CH3 group (see Figure 4, A.). Additions of OH groups at the 3- and 4-positions give rise to the catecholamines, which include dopamine, epinephrine, and norepinephrine. Shulgin's “2C” compounds (discussed below) lack an α-CH3 group, but contain MeO groups attached to the 2- and 5-position carbons.
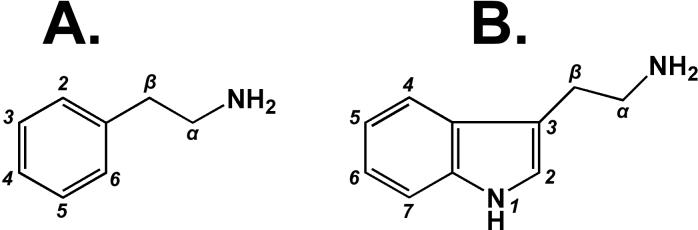
Figure 4.
Chemical structures, with position labels, for phenethylamine (A.) and tryptamine (B.). These two molecules form the chemical backbones for a range of hallucinogenic compounds discussed in this review.
The naturally occurring compound mescaline, an alkaloid isolated from the dumpling peyote cactus, has served as the lead compound in the development of structure-activity relationships (SAR) for the phenethylamines [88-93]. A major finding of hallucinogenic phenethylamine SAR is that increasing the length of the alkyl group on the 4-position oxygen atom increases potency of the resultant compound (as compared to mescaline, the reference standard). An important result of these efforts has been the design of relatively selective and potent compounds such as DOI and DOB, which have been widely used in vitro and in vivo to probe hallucinogen mechanisms of action. Further information on phenethylamine SAR is largely beyond the scope of this review, but the interested reader is directed to the erudite reviews of this subject by Jacob and Shulgin [94] and by Nichols [95].
As seen in Table 1, the selective phenethylamines DOI, DOM and DOB possess low nanomolar affinities for 5-HT2A receptors, and appreciably higher (on the order of 1,000-fold) affinities for 5-HT1A receptors. The selectivity of these compounds for 5-HT2A over 5-HT2C receptors is typically less than 10-fold, and some of the two carbon phenethylamine homologues of mescaline lacking the alpha-methyl group, such as 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7), actually display higher binding affinity for 5-HT2C receptors. Thus, although these drugs may be accurately described as 5-HT2-selective agents, there is generally reasonably promiscuous binding within the 5-HT2 receptor family. The potency of these compounds (with the exception of mescaline) also tends to be quite high, and the duration of action of these agents can last more than a day.
Table 1.
Binding affinities (Ki, nM) of various hallucinogenic phenethylamines mentioned in the text at selected central serotonin sites, and estimated hallucinogenic potency and duration of action (following oral administration) in man. Where possible, affinities are listed as determined by competition binding versus an agonist radioligand. Affinities for receptors in the inactive conformation (determined by saturation binding assays versus an antagonist radioligand) are generally lower (higher Ki values) for all compounds, and are indicated in the table by a superscripted asterisk. Dashes in cells denote no data on affinity at that receptor. Full references to the source articles, denoted by superscripted numbers, can be found in the References section.
| DRUG | 5-HT1A | 5-HT2A | 5-HT2C | Hallucinogenic potency in man | Duration of action in man |
|---|---|---|---|---|---|
| Mescaline | - | 20,000 167 | - | 200 − 400 mg 93 | 10 − 12 h 93 |
| DOI | 3,843 168 | 0.65 168 | 5.37 173 | 1.5 − 3.0 mg 93 | 16 − 30 h 93 |
| DOM | 7,267 169 | 21.00 170 | 41.68 171 | 3.0 − 10 mg 93 | 14 − 20 h 93 |
| DOB | 3,770 172 | 1.17 168 | 152.00 170 | 1.0 − 3.0 mg 93 | 18 − 30 h 93 |
| 2C-T-7 | 1,170 28,* | 120.00 28,* | 39.00 28,* | 10 − 30 mg 93 | 8 − 15 h 93 |
The 5-HT2A and 5-HT2C receptors are extremely similar in terms of structure and function, and studies of the biochemical efficacies of a series of tryptamine and phenethylamine hallucinogens at these receptors indicated that all of these compounds tend to act as partial agonists at the 5-HT2C receptor [96–97]. The majority of drug discrimination studies with these compounds have involved the training of DOI or DOM. Across multiple species, it has been shown that, in agreement with studies in humans, these hallucinogens generalize with one another [98-99]. Furthermore, antagonist correlation analysis has determined that the stimulus effects of phenethylamine hallucinogens are mediated by agonist activity at 5-HT2A receptors and are modulated by agonist activity at 5-HT2C receptors only rarely [100]. A specific example of this generalization is illustrated by the finding that the selective 5-HT2A antagonist M100907, but not the selective 5-HT2C antagonist SB 200,646, blocked the discriminative stimulus effects of DOI in the rat [101].
Head twitch studies involving injection of DOM and 2C-T-7 in the mouse have indicated pronounced antagonism of this effect by selective 5-HT2A antagonists such as M100907 [28], and mutant mice lacking this receptor do not exhibit the HTR when administered DOI [102]. Similarly, repeated exposure to various stressors increases the density of 5-HT2A receptors in the cortex [103-107], and such increases in receptor density have been shown to augment DOI-induced head twitches in rats and mice [108]. This implied relationship between stress, overexpression of 5-HT2A receptors, and hypersensitivity to DOI-elicited HTR was explicitly demonstrated by exposure to repeated foot-shock and a consequent augmentation of DOI-stimulated twitch behavior in the mouse [109].
The phenethylamines are also noted for the rapid development of tolerance which develops upon their repeated administration. This hallucinogen-induced tachyphylaxis has primarily been studied in humans receiving LSD [110], but tolerance also develops to some behavioral effects of phenethylamine hallucinogens amenable to study in animals, such as DOM-elicited HTR [111], and mescaline- and DOM-induced limb jerks [112]. The mechanism underlying such tolerance appears to be downregulation of 5-HT2A receptors, as decreases in 5-HT2A receptor density have been demonstrated in rats receiving chronic administration of hallucinogenic drugs [111, 113–115]. These changes in receptor expression have sometimes been correlated with changes in behavior. In this regard, repeated administration of DOM decreased both the HTR and 5-HT2A receptor density [111]. The neuroendocrine response to DOB was attenuated over one week of repeated administration, and at the end of this drug regimen a significant decrement in 5-HT2 receptor density was quantified [115]. Despite the high affinity of the phenethylamine hallucinogens for 5-HT2C receptors, most reports indicate that behaviors mediated by these receptors are unaltered by chronic drug administration [116]. As might be expected, the discriminative stimulus effects of phenethylamine hallucinogens are also subject to tolerance development. In the first demonstration of this phenomenon, tolerance was observed to the discriminative stimulus effects of DOI in animals treated with DOI for 8 days, although the discriminative performance of animals treated chronically with vehicle did not change significantly during the same time interval [117]. In these same studies, it was observed that repeated treatment with DOI decreased the density of 5-HT2A receptors, which was interpreted as a mechanism for the development of tolerance to the discriminative stimulus effects of DOI [117]. In keeping with the literature described above, chronic DOI treatment did not cause a downregulation of 5-HT2C receptors [117].
Tryptamines
The basic structure for all the hallucinogenic tryptamines is derived from tryptophan, which serves as an essential amino acid in some animals. The core structure is composed of a double indole ring system with an aminoethyl at the 3-position. Decarboxylation of the acid on the beta carbon converts tryptophan to tryptamine. The biosynthesis of various endogenous tryptamines proceeds through differential modification of the tryptophan structure. For example, serotonin biosynthesis commences with hydroxylation of the 5-position by typhtophan hydroxylase and then proceeds to decarboxylation of the β-carbon by amino acid decarboxylase. The biosynthesis of other endogenous tryptamines such as melatonin proceeds through a different metabolic sequence. Since all tryptamines contain the basic indole ring, they are all structurally similar to serotonin. This double ring system contains seven positions that are open to chemical modification (see Figure 4, B.). However, the majority of medicinal chemistry efforts have thus far focused on modification of the 4- and 5-positions. One reason for this is because it has been shown that modification of either the 6- or 7-positions significantly reduce the psychoactive effects of the resulting compound [118]. Therefore, the hallucinogenic tryptamines can be largely divided into three broad categories based on modification to the 4- or 5-position on the indole ring, or a lack of modification to either position.
Leaving the indole ring unsubstituted, but adding two methyl groups on the terminal amine of the aminoethyl group, results in DMT which serves as a prototypical member of the ring-unmodified tryptamines. This hallucinogenic agent has been known for thousands of years to be naturally occurring in the Banisteriopsis caapi plant and has been extensively used in the form of a tea called ayahuasca that is consumed during religious ceremonies of the indigenous people of the pan-Amazonian delta [119]. Double methylation and hydroxylation of the 4-position produces 4-hydroxy-N,N-dimethyltryptamine (4-OH-DMT, psilocin) which can serve as the prototypical agent of the 4-position modified tryptamines. Psilocin is the dephosphorylated active metabolite of the naturally occurring tryptamine psilocybin which is found in certain types of hallucinogenic mushrooms. It is thus important to recognize that, although psilocybin is the more commonly consumed agent, metabolic events within the body occur to biotransform this compound to psilocin, which mediates the majority of the drug effects in vivo. The prototypical compound chosen for the 5-position modified tryptamines is 5-methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT). 5-MeO-DIPT or “Foxy Methoxy” is a synthetic designer drug which has largely come under scientific interest after its synthesis and description by Shulgin [118]. It is produced by the addition of two isopropyl groups to the terminal nitrogen of the aminoethyl group and methoxylation of the 5-position of the indole ring. The receptor and behavioral pharmacology of these different subclasses of the tryptamines will be discussed with principal comparisons made between these prototypical compounds.
Although the structural similarity of the tryptamines to serotonin engenders significant activity at serotonergic receptors across the chemical class, many tryptamines also bind and activate non-serotonergic receptors as well. Furthermore, the tryptamines have relatively lower affinity for the 5-HT2A receptors than do either the phenethylamines or the ergolines. Even within the original paper [77] the tryptamines tested fell within a range of 207 nM (5-MeO-DMT) and 462 nM (DMT) affinity for the 5-HT2A receptor, as compared to the hallucinogenic phenethylamines which largely fell within a range of 6 nM (DOI) to 162 nM (DOM). However, LSD did display the highest affinity for the 5-HT2A receptor at 0.95 nM [77]. One limitation of this work was that the hallucinogens were tested in competition with the antagonist ketanserin, and, as described above, this may profoundly influence apparent affinity [78]. As Table 2 illustrates, more recent research has demonstrated that the tryptamines DMT, psilocin, and 5-MeO-DIPT have 230 nM, 5,620 nM, and 25 nM affinity for the 5-HT2A receptor, respectively. This compares to the phenethylamines DOI, DOM, DOB, and 2C-T-7 which have respective affinities for the 5-HT2A receptor of 0.65 nM, 21 nM, 1.17 nM, and 120 nM (Table 1). Thus, the tryptamines tend to have lower affinity for the 5-HT2A receptor than do the phenethylamines.
Table 2.
Binding affinities (Ki, nM) of various hallucinogenic tryptamines and ergolines mentioned in the text at selected central serotonin sites, and estimated hallucinogenic potency and duration of action (following oral administration) in man. Where possible, affinities are listed as determined by competition binding versus an agonist radioligand. Affinities for receptors in the inactive conformation (determined by saturation binding assays versus an antagonist radioligand) are generally lower (higher Ki values) for all compounds, and are indicated in the table by a superscripted asterisk. Dashes in cells denote no data on affinity at that receptor, while N.A. signifies a lack of hallucinogenic activity (but see text for more on this). Full references to the source articles, denoted by superscripted numbers, can be found in the References section.
| DRUG | 5-HT1A | 5-HT2A | 5-HT2C | Hallucinogenic potency in man | Duration of action in man |
|---|---|---|---|---|---|
| Psilocin | 49.00 174 | 25.00 174 | 10.00 174 | 10 − 20 mg 118 | 3 − 6 h 118 |
| LSD | 1.10 175 | 3.50 175 | 5.50 175 | 60 − 200 μg 118 | 8 − 12 h 118 |
| Lisuride | 0.15 176 | 1.40 177 | 5.30 177 | N.A. | N.A. |
| DMT | 119.51 178 | 230.27 178,* | - | > 350 mg 118 | ≤ 1 h 118 |
| 5-MeO-DIPT | 35.00 29,* | 5,620 29,* | 1,700 29,* | 6 − 12 mg 118 | 4 − 8 h 118 |
In the original correlation [77], LSD had the highest affinity for the 5-HT2A receptor and the highest potency for hallucinogenic effect in humans. It is still the case that LSD has the highest potency for hallucinogenic effects, however, as Tables 1 and 2 show, when the test compounds are placed in competition with agonists, the phenethylamines DOI and DOB have affinities for the 5-HT2A receptor comparable to that of LSD. Furthermore, one of the main lines of evidence that discredited the idea that hallucinogenesis is mediated by suppression of dorsal raphe firing was that the putatively non-hallucinogenic compound lisuride also suppresses neuronal firing in this region [73]. As can been seen in Table 2, lisuride has comparable or in some cases considerably higher affinity for the 5-HT2A receptor than some hallucinogenic tryptamines and phenethylamines. In addition, the phenethylamine hallucinogen mescaline showed very low affinity for the 5-HT2A receptor, however, a full description of this work must come with the caveat that mescaline binding was measured against the bovine 5-HT2A receptor. It is possible that mescaline's lack of affinity for the receptor is due to some species difference rather than a lack of affinity for the human 5-HT2A receptor. Furthermore, while mescaline is hallucinogenic, it displays relatively low potency via the oral route.
Some of the evidence cited above may seem to challenge the role of 5-TH2A receptors in hallucinogenesis, but the concept of agonist-specific signaling might have some explanatory power here. Agonist-specific signaling is a relatively novel concept in pharmacology that posits that while two drugs may be agonists at the same receptor, they may nonetheless selectively activate different functional effects. Recent work by Gonzales-Maeso and colleagues has shown that hallucinogenic and putatively non-hallucingenic 5-HT2A agonists activate a different set of genes [102, 120]. Although these observed genetic changes are not proposed as the mechanism for the hallucinogen-like effects of the various agonists, they serve as markers for activation of a differential set of second messenger systems. Furthermore, these hallucinogenic agonists could be distinguished from purportedly non-hallucinogenic agonists in vivo by the mouse head twitch assay. Using a series of toxins [120], Gonzales-Maseo and associates further demonstrated in vitro that hallucinogenic 5-HT2A agonists may induce a conformation whereby the receptor can couple to a Gi protein in addition to the Gq/11 protein that is classically considered to be coupled to the 5-HT2A receptor. Additionally, a recent review article cited several publications pointing to the possibility of functional selectivity at the 5-HT2A receptor [14]. This work is still in the early stages and thus it is unclear whether this will explain the effects of hallucinogenic versus purportedly non-hallucinogenic 5-HT2A agonists. Therefore, given that hallucinogenic compounds display widely divergent affinities for the 5-HT2A receptor, that some putative non-hallucinogens display high affinity for the receptor, that some hallucinogens display a lack of affinity for the receptor, and that a correlation can be drawn between hallucinogenic potency in man and affinity for serotonin receptor subtypes not known to have a role in hallucinogenesis, it seems plausible to speculate that the 5-TH2A receptor may not be the sole mediator of hallucinogenic effects. However, this view contrasts with the overwhelming body of behavioral pharmacology that implicates a role for the 5-HT2A receptor. Therefore, while the 5-HT2A receptor is clearly a critical site of action for the hallucinogenic drugs, we may speculate that it is not the only important mediator of hallucinogenesis.
But if the 5-HT2A receptor is not the sole mediator of hallucinogenic effects, then what other receptors may be involved? The early hypothesis of suppression of dorsal raphe firing by Aghajanian and colleagues was based work carried out primarily with tryptamines and the ergoline LSD [70 – 72]. Although this hypothesis was eventually abandoned, the evidence that the tryptamines suppress firing of the dorsal raphe neurons is still widely accepted. Indeed, it was ultimately discovered that this suppressive effect was mediated by pre-synaptic autoinhibitory feedback initiated by stimulation of somatodendritic 5-HT1A receptors [121–122]. As can be seen in Table 1, the phenethylamines have essentially no biologically relevant affinity for the 5-HT1A receptor, and it is therefore not surprising that they do not share this effect on raphe neurons.
Thus, if the hallucinogen-like effects of the tryptamines mediated, at least in part, by 5-HT1A receptors, then the behavioral and discriminative stimulus effects in animal models should also be mediated by the 5-HT1A receptor. There is some evidence to support this hypothesis. It has been found that the rank order of potency for compounds which substitute in rats trained to discriminate 5-MeO-DMT from saline correlates more tightly with their affinity for the 5-HT1A receptor than with their affinity for 5-HT2 receptors [123]. Furthermore, of the antagonists tested, only the selective 5-HT1A antagonist pindolol and the mixed 5-HT1A/2A antagonist metitepine could fully block the 5-MeO-DMT cue [124]. Consistent with this finding, the 5-HT1A selective antagonists pindolol and WAY100635 antagonized the discriminative stimulus effect of 5-MeO-DMT, whereas the 5HT2 receptor selective antagonist pirenperone did not [124]. Furthermore, the 5-HT1A selective agonist 8-OH-DPAT fully substituted for 5-MeO-DMT, whereas, the phenethylamine DOM (which lacks relevant affinity at 5-HT1A) only partially substituted [124]. The message of these studies seems to be that the discriminative stimulus properties of 5-MeO-DMT are primarily mediated through the 5-HT1A receptor.
Furthermore, a recent study with rats trained to discriminate LSD found that 5-HT1A agonists enhanced stimulus control by LSD and this effect could be blocked by specific 5-HT1A receptor antagonists [125]. A recent study by Porter and colleagues [126] tested various hallucinogens in CHO-K1 cells transfected with human 5-HT2A, 5-HT2B, and 5-HT2C receptors, and reported that the tryptamines tested displayed a rank order of potency of 5-HT2B > 5-HT2C > 5-HT2A whereas the phenethylamines tested displayed a rank order of potency of 5-HT2A > 5-HT2B > 5-HT2C. This work further supports the existing evidence that, in contrast to the phenethylamines, typtamines preferentially bind to receptors other than the 5-HT2A receptor. Additionally, it has been shown that DMT is largely inactive in the mouse head twitch assay [29]. However, in the same study it was shown that despite at least a 100-fold selectivity in binding for the 5-HT1A receptor over the 5-HT2A or 5-HT2C receptors, 5-MeO-DIPT elicited a robust HTR [29]. Furthermore, it was demonstrated that while the discriminative stimulus effects of 5-MeO-DIPT were partially but surmountably blocked by the 5-HT1A receptor antagonist WAY100635, they were completely and insurmountably blocked by the 5-HT2A receptor antagonist M100907 [29]. The strongest evidence that the 5-HT1A receptor cannot by itself mediate hallucinogen-like effects is that there are no known agonists for the 5-HT1A receptor that function as hallucinogens in man. Therefore, we suggest that while the 5-HT2A receptor is likely to be the final common mechanism of hallucinogenic effects, the tryptamines and ergolines differ from the phenethylamines in that their subjective and behavioral effects are clearly modified by activity at the 5-HT1A receptor.
The most convincing evidence of a role for the 5-HT1A receptor in the actions of hallucinogenic tryptamines would be a clinical study in which human subjects were administered such compounds in the presence and absence of a 5-HT1A antagonist. To our knowledge, no such studies have yet been undertaken. In fact, aside from the ethical difficulties inherent in such research, these experiments may turn out to be more difficult than anticipated. Previous authors have penned that the subjective effects of the hallucinogens in man, unlike other psychoactive compounds, are heavily modified by internal and external environmental variables such as mood, expectation, room lighting, and music [14, 93, 118]. Given the tightly controlled environment of laboratory animal testing, it may thus turn out to be the case that the animal models provide a more objective measure of the direct effects of these drugs. However, at least one recent study has been carried out in which the researchers tightly controlled the environment in psilocin was administered to human subjects [8]. This research points a way forward for appropriate design of future human research with hallucinogens, and may eventually allow for the assessment of the role 5-HT1A receptors play in the subjective effects of the tryptamines in man.
More recently, a role for the newly-discovered trace amine-associated receptors (TAAR) has been suggested by the relatively high affinity of tryptamine-containing hallucinogens such as LSD [127] and DMT [128-129] for these sites. More broadly, TAAR also bind substituted phenethylamines such as amphetamine, DOI and MDMA [127], perhaps suggesting a common pathway for the “hallucinogen-like” effects of all of these agents (observed only at high doses with amphetamine and MDMA). Despite this promise, significant obstacles currently exist in the field of TAAR research. For example, three distinct families of TAAR, consisting of up to 21 individual receptors, have been identified and characterized in human, chimpanzee, rat, and mouse, and significant interspecies differences have been discovered [130]. The density of these sites in native tissue is extremely low, this TAAR must be cloned and expressed for in vitro assays, and thus far, few stable expression systems have been developed [130]. Until these and other technical issues are resolved, the role of TAAR in hallucinogen-induced effects must be regarded as unknown.
LSD
LSD is an extremely potent compound, with typical active human doses ranging between 0.05 and 0.20 mg. The subjective effects resulting from LSD ingestion can last up to 12 hours and include alterations of mood, perceptual changes, and cognitive impairment [1, 14, 131–132]. Despite the capacity of LSD to induce profound perceptual changes, there is a lack of evidence demonstrating adverse physical consequences as a direct result of LSD administration. Indeed, there are no documented cases of death due to LSD overdose, however, one purported adverse consequence of LSD use is hallucinogen persisting perception disorder (HPPD), otherwise referred to as a “flashback”. This phenomena is defined (in part) as “the reexperiencing, following cessation of use of a hallucinogen, of one or more of the perceptual symptoms that were experienced while intoxicated with the hallucinogen” [133]. In a comprehensive review of 20 studies, Halpern and Pope (2003) note that while some studies have reported the absence of “major complications” following LSD administration [132], other studies have reported rates of “spontaneous recurrences of LSD reactions” as high as 33% [134]. Polysubstance use, lack of dosage information, an inability to reliably trigger the phenomenon in the laboratory, and preexisting psychological disorders among the subjects examined make estimating the incidence of HPPD due to LSD use problematic, however, when considering the sheer number of people who have ingested LSD however, the incidence of HPPD would seem to be quite low [16].
As with the phenethylamine and tryptamine hallucinogens previously described, LSD can also function as a discriminative stimulus in the rat, and the stimulus effects of this ergoline can be blocked by prior administration of serotonergic antagonists [98, 125]. Later, Glennon and colleagues implicated the 5-HT2 receptor in the mechanism of action of LSD and hallucinogenesis based upon a correlation between the affinities of several hallucinogens for the 5-HT2 receptor and hallucinogenic potency in man [135-136]. However, the subsequent discovery of a 5-HT2C receptor [137-138] with a high level of structural homology and functional similarity to the 5-HT2A receptor, and the demonstration that tryptamine and phenethylamine hallucinogens act as partial agonists at the 5-HT2C receptor [96-97] demanded consideration of this potential role of this serotonin receptor subtype as well. To address this question, a series of antagonists with varying selectivity for 5-HT2A and 5-HT2C receptors were used. Results of these experiments [100] determined that the in vivo potency of antagonists to block the stimulus cue of LSD is directly proportional to the in vitro affinity of those same antagonists for 5-HT2A receptors. The role of the 5-HT2A receptor in the stimulus effects of LSD is further buttressed by the complete blockade of the stimulus effects of LSD by the highly 5-HT2A-selective antagonist M100907 [139]. Other drug discrimination studies have specifically implicated the anterior cingulate cortex (ACC) in the stimulus effects of LSD, as LSD locally infused into ACC dose-dependently substituted for rats trained to discriminate systemically administered LSD from saline [140]. Additional, indirect support for a role of 5-HT2A receptors in LSD's mechanism of action stems from their anatomical location in the frontal cortex [141] an area containing a high density of 5-HT2A receptors [138, 142] and thought to play a significant role in hallucinogenesis and psychosis [143-146].
In one of the few studies where non-human primates were trained to discriminate a hallucinongen, the 5-HT2 receptor antagonists ketanserin and pirenperone largely failed to block the interoceptive effects of LSD in vervet monkeys [147]. Furthermore, the purported 5-HT2A specific agonist mescaline did not substitute for the LSD cue, whereas, the mixed 5-HT1A/2A agonist 5-MeO-DMT did fully substitute [147]. However, the putatively non-hallucinogenic compound lisuride did fully substitute for LSD, raising the possibility that the LSD cue was unrelated to any hallucinogenic effects [147]. Nevertheless, these data may suggest that, like the tryptamines, the discriminative stimulus properties of LSD may also be mediated, at least partially, through the 5-HT1A receptor.
With regard to lisuride, the designation of this compound as “non-hallucinogenic” is by no means well established. Animals trained to discriminate LSD generalize their responding to lisuride [148–149], which has lead to the classification of this agent as a “false positive” under these procedures. Indeed, the substitution of lisuride for LSD has long noted as a deficiency of the drug discrimination procedure, at least in terms of hallucinogen-induced stimulus control. But what is the evidence that lisuride is without hallucinogen action in man? Lisuride has been investigated as an anti-migraine medication, and as a therapeutic for Parkinson's disease. Several reports of the effects of lisuride in man thus appeared in the clinical literature in the early 1980s, and numerous such reports indicate that lisuride elicited toxic side effects including visual hallucination, reduced awareness, delusions, auditory hallucination, euphoria, morbid jealousy and paranoid ideation [150–155]. This side effect profile is not entirely inconsistent with the psychological effects of some hallucinogens. Nevertheless, the hallucinatory effects of lisuride, when present, are sometimes slow in onset, and at least one report explicitly states that no LSD-like effects have been observed in healthy volunteers [156]. Thus, the hallucinogenic status of this most interesting ergoline will likely remain controversial.
While it is well established that 5-HT2A receptor stimulation is a necessary prerequisite for the effects of LSD [14, 79, 100, 131] the biochemical and signaling pathways responsible for producing LSD's extraordinary effects remain largely unknown. The 5-HT2 receptors (including the 5-HT2A receptor) are G-protein coupled receptors. These membrane bound receptors have seven transmembrane spanning domains and are classically linked to the activation of phospholipase C (PLC) via an activation of Gq/11. This activation results in the hydrolysis of phosphatidylinositol 4,5-biphosphate (PIP2) and the generation of 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). The activation of IP3 and DAG lead to the release of intracellular Ca2+, and the activation of protein kinase C respectively [157]. Because 5-HT2A activation activates PLC and phosphoinositide hydrolysis (PI) in most tissues, it has been assumed this is the relevant pathway for LSD signaling. However, there is no correlation between the capacity of a compound to substitute for LSD in the drug discrimination assay and PI hydrolysis [158] and LSD itself stimulates PI hydrolysis only weakly [96]. These results highlight the distinct differences between LSD and 5-HT with regard to activation of PLC and other signaling pathways. Indeed, different ligands for 5-HT2A receptors have been shown to differentially activate 5-HT2A signal transduction pathways, including other phospholipase subtypes [159-160].
Activation of 5-HT2A receptors also leads to activation of the phospholipase A2 (PLA2) signaling pathway and subsequent arachidonic acid release (AA) [157]. Relative to the endogenous ligand 5-HT, in NIH3T3−5HT2A cells, LSD stimulates the PLA2 pathway to a greater extent than the PLC pathway. The significance of this finding with regards to LSD's mechanism of action is unclear though, as other hallucinogens (e.g. psilocin and 5-MeO-DMT) do not share this property (e.g. they stimulate PLC to a greater degree than PLA2) [159]. Lastly, 5-HT2A receptors couple to the phospholipase D (PLD) signaling pathway [157]. This effect may be cell type specific [161] and has not been investigated with regards to LSD. Thus, the role of PLD signaling in LSD's mechanism of action remains unclear. Some of this uncertainty may be due to the previously discussed phenomenon of agonist-specific signaling via the 5-HT2A receptor. Recent studies with LSD (among other hallucinogens) support this hypothesis [120]. Theoretical data from mathematical modeling of drug-receptor interactions and experimental observations suggest that G protein-coupled receptors (such as 5-HT2A) may assume multiple conformations in response to agonist binding [102, 162-164], which in turn preferentially activate specific signaling pathways [165-166].
Summary and conclusions
Current methods in behavioral pharmacology and neuroscience are finally beginning to chip away at the mystical façade that has defined the hallucinogens for too long. With the identification of exploitable SAR for these compounds, hypothesis-driven chemical syntheses have allowed the development of homologous compounds with specific binding at relevant 5-HT receptors. Study of the effects of these drugs on conditioned (drug discrimination) and unconditioned (HTR) behaviors have enabled scientists to bring these interesting compounds out of the counterculture and into the laboratory. The resulting data have indicated that the 5-HT2A receptor is a critically important site of action for the hallucinogens, and have directed further attention towards understanding the modulatory roles of 5-HT2C (for the phenethylamines) and 5-HT1A receptors (for the tryptamines). Advanced genetic and molecular biological techniques have identified the possibility that agonist-specific signaling is a factor in the mechanisms of action among these drugs, an idea which promises to capture research attention for years to come. All of these developments leave us on much firmer ground with regards to the study of hallucinogens than might have been imagined a generation before, and clinical interest in the therapeutic potential of these compounds is once again beginning to emerge. It is thus ironic to see that the hallucinogens – long claimed to be scientifically intractable – have yielded so much information in such a comparatively short time, and that the often desired resumption of human research with these agents might indeed be fit to commence thanks to controlled preclinical studies of these drugs.
Acknowledgements
The authors thank Sasha and Ann Shulgin for inspiration in the writing of this review, and acknowledge the generous funding provided by USPHS Grant DA020645 and by the College on Problems of Drug Dependence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hofmann A. Psychotomimetic drugs; chemical and pharmacological aspects. Acta Physiol Pharmacol Neerl. 1959;8:240–58. [PubMed] [Google Scholar]
- 2.Dyck E. Flashback: psychiatric experimentation with LSD in historical perspective. Can J Psychiatry. 2005;50:381–8. doi: 10.1177/070674370505000703. [DOI] [PubMed] [Google Scholar]
- 3.Gouzoulis-Mayfrank E, Hermle L, Thelen B, Sass H. History, rationale and potential of human experimental hallucinogenic drug research in psychiatry. Pharmacopsychiatry. 1998;31(Suppl 2):63–8. doi: 10.1055/s-2007-979348. [DOI] [PubMed] [Google Scholar]
- 4.Hollister LE. Drug-induced psychoses and schizophrenic reactions: a critical comparison. Annals of the New York Academy of Sciences. 1962;96:80–92. doi: 10.1111/j.1749-6632.1962.tb50103.x. [DOI] [PubMed] [Google Scholar]
- 5.Carter OL, Burr DC, Pettigrew JD, Wallis GM, Hasler F, Vollenweider FX. Using psilocybin to investigate the relationship between attention, working memory, and the serotonin 1A and 2A receptors. J Cog Neurosci. 2005;17:1497–508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- 6.Carter OL, Pettigrew JD, Burr DC, Alais D, Hasler F, Vollenweider FX. Psilocybin impairs high-level but not low-level motion perception. Neuroreport. 2004;15:1947–51. doi: 10.1097/00001756-200408260-00023. [DOI] [PubMed] [Google Scholar]
- 7.Carter OL, Pettigrew JD, Hasler F, Wallis GM, Liu GB, Hell D, et al. Modulating the rate and rhythmicity of perceptual rivalry alternations with the mixed 5-HT2A and 5-HT1A agonist psilocybin. Neuropsychopharmacology. 2005;30:1154–62. doi: 10.1038/sj.npp.1300621. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology. 2006;187:268–83. doi: 10.1007/s00213-006-0457-5. discussion 84−92. [DOI] [PubMed] [Google Scholar]
- 9.Vollenweider FX, Leenders KL, Scharfetter C, Maguire P, Stadelmann O, Angst J. Positron emission tomography and fluorodeoxyglucose studies of metabolic hyperfrontality and psychopathology in the psilocybin model of psychosis. Neuropsychopharmacology. 1997;16:357–72. doi: 10.1016/S0893-133X(96)00246-1. [DOI] [PubMed] [Google Scholar]
- 10.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9:3897–902. doi: 10.1097/00001756-199812010-00024. [DOI] [PubMed] [Google Scholar]
- 11.Strassman RJ. Human psychopharmacology of N,N-dimethyltryptamine. Behavioural Brain Research. 1996;73:121–4. doi: 10.1016/0166-4328(96)00081-2. [DOI] [PubMed] [Google Scholar]
- 12.Strassman RJ, Qualls CR. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Archives of General Psychiatry. 1994;51:85–97. doi: 10.1001/archpsyc.1994.03950020009001. [DOI] [PubMed] [Google Scholar]
- 13.Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Archives of General Psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- 14.Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–81. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Overton DA. Discriminative control of behavior by drug states. In: Thompson T, Pickens R, editors. Stimulus Properties of Drugs. Appleton-Century-Crofts; New York: 1971. pp. 87–110. [Google Scholar]
- 16.Corne SJ, Pickering RW, Warner BT. A method for assessing the effects of drugs on the central actions of 5-hydroxytryptamine. Brit J Pharmacol. 1963;20:106–120. doi: 10.1111/j.1476-5381.1963.tb01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corne SJ, Pickering RW. A possible correlation between drug-induced hallucinations in man and a behavioral response in mice. Psychopharmacologia. 1967;11:65–78. doi: 10.1007/BF00401509. [DOI] [PubMed] [Google Scholar]
- 18.Peroutka SJ, Lebovitz RM, Snyder SH. Two distinct central serotonin receptors with different physiological functions. Science. 1981;212:827–829. doi: 10.1126/science.7221567. [DOI] [PubMed] [Google Scholar]
- 19.Colpaert FC, Janssen PAJ. The head twitch response to intraperitoneal injection of 5-hydroxytryptophan in the rat: Antagonist effects of purported 5-hydroxytryptamine antagonists and of pirenperone, an LSD antagonist. Neuropharmacology. 1983;22(8):993–1000. doi: 10.1016/0028-3908(83)90215-0. [DOI] [PubMed] [Google Scholar]
- 20.Green AR, O'Shaughnessy K, Hammond M, Schachter M, Grahame-Smith DG. Inhibition of 5-hydroxytryptamine-mediated behaviours by the putative 5-HT2 receptor antagonist pirenperone. Neuropharmacology. 1983;22:573–578. doi: 10.1016/0028-3908(83)90147-8. [DOI] [PubMed] [Google Scholar]
- 21.Goodwin GM, Green AR. A behavioural and biochemical study in mice and rats of putative selective agonists and antagonists for 5-HT1 and 5-HT2 receptors. Br J Pharmacol. 1985;84(3):743–53. doi: 10.1111/j.1476-5381.1985.tb16157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darmani NA, Martin BR, Glennon RA. Withdrawal from chronic treatment with (±)-DOI causes supersensitivity to 5-HT2 receptor-induced head-twitch behavior in mice. Eur J Pharmacol. 1990;186:115–118. doi: 10.1016/0014-2999(90)94066-7. [DOI] [PubMed] [Google Scholar]
- 23.Darmani NR, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- 24.Darmani NA, Martin BR, Glennon RA. Behavioral evidence for differential adaptation of the serotonergic system after acute and chronic treatment with (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) or ketanserin. J Pharmacol Exp Ther. 1992;262(2):692–698. [PubMed] [Google Scholar]
- 25.Fantegrossi WE, Kiessel CL, Leach PT, Van Martin C, Karabenick RL, Chen X, Ohizumi Y, Ullrich T, Rice KC, Woods JH. Nantenine: an antagonist of the behavioral and physiological effects of MDMA in mice. Psychopharmacology. 2004;173(3−4):270–7. doi: 10.1007/s00213-003-1741-2. [DOI] [PubMed] [Google Scholar]
- 26.Lucki I, Nobler MS, Frazer A. Differential actions of serotonin antagonists on two behavioral models of serotonin receptor activation in the rat. J Pharmacol Exp Ther. 1984;228:133–139. [PubMed] [Google Scholar]
- 27.Handley SL, Singh L. Neurotransmitters and shaking behavior: More than a “gut bath” for the brain. Trends Pharmacol Sci. 1986;7:324–328. [Google Scholar]
- 28.Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology. 2005;181(3):496–503. doi: 10.1007/s00213-005-0009-4. [DOI] [PubMed] [Google Scholar]
- 29.Fantegrossi WE, Harrington AW, Kiessel CL, Eckler JR, Rabin RA, Winter JC, Coop A, Rice KC, Woods JH. Hallucinogen-like actions of 5-methoxy-N,N-diisopropyltryptamine in mice and rats. Pharmacol Biochem Behav. 2006;83(1):122–9. doi: 10.1016/j.pbb.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Ortmann R, Biscoff S, Radeke E, Bueche O, Delini-Stula A. Correlation between different measures of antiserotonin activity of drugs. Naunyn Schmiedeberg's Arch Pharmacol. 1982;321:265–270. doi: 10.1007/BF00498511. [DOI] [PubMed] [Google Scholar]
- 31.Aceto MD, McKean DB, Pearl J. Effects of opiates and opiate antagonists on the Straub tail reaction in mice. Br J Pharmacol. 1969;36(2):225–39. doi: 10.1111/j.1476-5381.1969.tb09500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dursun SM, Handley SL. Similarities in the pharmacology of spontaneous and DOI-induced head-shakes suggest 5-HT2A receptors are active under physiological conditions. Psychopharmacology. 1996;128:198–205. doi: 10.1007/s002130050125. [DOI] [PubMed] [Google Scholar]
- 33.Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT)2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- 34.Darmani NA. Cocaine and selective monoamine uptake blockers (sertraline, nisoxetine, and GBR 12935) prevent the d-fenfluramine-induced head-twitch response in mice. Pharmacol Biochem Behav. 1998;60(1):83–90. doi: 10.1016/s0091-3057(97)00548-0. [DOI] [PubMed] [Google Scholar]
- 35.Skinner BF. The Behavior of Organisms. Appleton-Century-Crofts; New York: 1938. [Google Scholar]
- 36.Ferster CB, Skinner BF. Schedules of Reinforcement. Appleton-Century-Crofts; New York: 1957. [Google Scholar]
- 37.Goldberg SR, Morse WH, Goldberg DM. Behavior maintained under a second-order schedule by intramuscular injection of morphine or cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1976;199:278–286. [PubMed] [Google Scholar]
- 38.Carroll ME, Krattiger KL, Gieske D, Sadoff DA. Cocaine-base smoking in rhesus monkeys: reinforcing and physiological effects. Psychopharmacology. 1990;102:443–50. doi: 10.1007/BF02247123. [DOI] [PubMed] [Google Scholar]
- 39.Gomez TH, Roache JD, Meisch RA. Orally delivered alprazolam, diazepam, and triazolam as reinforcers in rhesus monkeys. Psychopharmacology. 2002;161(1):86–94. doi: 10.1007/s00213-002-1019-0. [DOI] [PubMed] [Google Scholar]
- 40.Yanagita T, Deneau GA, Seevers MH. Committee on Drug Addiction and Narcotics. NRCNAS; Ann Arbor, MI: 1963. Methods for studying psychogenic dependence to opiates in the monkey. [Google Scholar]
- 41.Schuster CR, Thompson T. Committee on Drug Addiction and Narcotics. NRCNAS; Ann Arbor, MI: 1963. A technique for studying self-administration of opiates in Rhesus monkeys. [Google Scholar]
- 42.Carroll ME. Pharmacological and behavioral treatment of cocaine addiction: animal models. NIDA Res Monogr. 1994;145:113–130. [PubMed] [Google Scholar]
- 43.Wojnicki FH, Bacher JD, Glowa JR. Use of subcutaneous vascular access ports in rhesus monkeys. Lab Anim Sci. 1994;44(5):491–494. [PubMed] [Google Scholar]
- 44.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use, 1975−2005. Volume II: College students and adults ages 19−45 (NIH Publication No. 06−5884) National Institute on Drug Abuse; Bethesda, MD: 2006. [Google Scholar]
- 45.Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacologia. 1969;16(1):30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- 46.Yanagita T. Intravenous self-administration of (−)-cathinone and 2-amino-1-(2,5-dimethoxy-4-methyl)phenylpropane in rhesus monkeys. Drug Alcohol Depend. 1986;17(2−3):135–41. doi: 10.1016/0376-8716(86)90004-9. [DOI] [PubMed] [Google Scholar]
- 47.Lamb RJ, Griffiths RR. Self-injection of d,1−3,4-methylenedioxymethamphetamine (MDMA) in the baboon. Psychopharmacology. 1987;91(3):268–72. doi: 10.1007/BF00518175. [DOI] [PubMed] [Google Scholar]
- 48.Beardsley PM, Balster RL, Harris LS. Self-administration of methylenedioxymethamphetamine (MDMA) by rhesus monkeys. Drug Alcohol Depend. 1986;18(2):149–57. doi: 10.1016/0376-8716(86)90047-5. [DOI] [PubMed] [Google Scholar]
- 49.Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G. 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology. 2002;161:356–364. doi: 10.1007/s00213-002-1021-6. [DOI] [PubMed] [Google Scholar]
- 50.Lile JA, Ross JT, Nader MA. A comparison of the reinforcing efficacy of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) with cocaine in rhesus monkeys. Drug Alcohol Depend. 2005;78(2):135–40. doi: 10.1016/j.drugalcdep.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Schenk S, Gittings D, Johnstone M, Daniela E. Development, maintenance and temporal pattern of self-administration maintained by ecstasy (MDMA) in rats. Psychopharmacology. 2003;169(1):21–7. doi: 10.1007/s00213-003-1407-0. [DOI] [PubMed] [Google Scholar]
- 52.Trigo JM, Panayi F, Soria G, Maldonado R, Robledo P. A reliable model of intravenous MDMA self-administration in naive mice. Psychopharmacology. 2006;184(2):212–20. doi: 10.1007/s00213-005-0229-7. [DOI] [PubMed] [Google Scholar]
- 53.Sannerud CA, Kaminski BJ, Griffiths RR. Intravenous self-injection of four novel phenethylamines in baboons. Behav Pharmacol. 1996;7(4):315–323. doi: 10.1097/00008877-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Griffiths RR, Winger G, Brady JV, Snell JD. Comparison of behavior maintained by infusions of eight phenylethylamines in baboons. Psychopharmacology. 1976;50(3):251–8. doi: 10.1007/BF00426841. [DOI] [PubMed] [Google Scholar]
- 55.Poling A, Bryceland J. Voluntary drug self-administration by nonhumans: a review. J Psychedelic Drugs. 1979;11:185–190. doi: 10.1080/02791072.1979.10472103. [DOI] [PubMed] [Google Scholar]
- 56.Fantegrossi WE, Woods JH, Winger G. Transient reinforcing effects of phenylisopropylamine and indolealkylamine hallucinogens in rhesus monkeys. Behav Pharmacol. 2004;15(2):149–57. doi: 10.1097/00008877-200403000-00007. [DOI] [PubMed] [Google Scholar]
- 57.Young AM, Herling S, Woods JH. History of drug exposure as a determinant of drug self-administration. NIDA Res Monogr. 1981;37:75–88. [PubMed] [Google Scholar]
- 58.Balster RL, Woolverton WL. Continuous-access phencyclidine self-administration by rhesus monkeys leading to physical dependence. Psychopharmacology. 1980;70(1):5–10. doi: 10.1007/BF00432363. [DOI] [PubMed] [Google Scholar]
- 59.Moreton JE, Meisch RA, Stark L, Thompson T. Ketamine self-administration by the rhesus monkey. J Pharmacol Exp Ther. 1977;203(2):303–9. [PubMed] [Google Scholar]
- 60.Nicholson KL, Jones HE, Balster RL. Evaluation of the reinforcing and discriminative stimulus properties of the low-affinity N-methyl-D-aspartate channel blocker memantine. Behav Pharmacol. 1998;9(3):231–43. [PubMed] [Google Scholar]
- 61.Tanda G, Munzar P, Goldberg SR. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3(11):1073–4. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- 62.Tanda G, Goldberg SR. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms – a review of recent preclinical data. Psychopharmacology. 2003;169(2):115–34. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- 63.Kelleher RT, Morse WH. Determinants of the specificity of behavioral effects of drugs. Ergeb Physiol. 1968;60:1–56. doi: 10.1007/BFb0107250. [DOI] [PubMed] [Google Scholar]
- 64.Spealman RD, Goldberg SR. Drug self-administration by laboratory animals: control by schedules of reinforcement. Annu Rev Pharmacol Toxicol. 1978;18:313–39. doi: 10.1146/annurev.pa.18.040178.001525. [DOI] [PubMed] [Google Scholar]
- 65.Gaddum JH. Antagonism between lysergic acid diethylamide and 5-hydroxytryptamine. J Physiol. 1953;121(1):15P. [PubMed] [Google Scholar]
- 66.Gaddum JH, Hameed KA. Drugs which antagonize 5-hydroxytryptamine. Br J Pharmacol. 1954;9(2):240–8. doi: 10.1111/j.1476-5381.1954.tb00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cerletti A, Rothlin E. Role of 5-hydroxytryptamine in mental diseases and its antagonism to lysergic acid derivatives. Nature. 1955;176(4486):785–6. doi: 10.1038/176785a0. [DOI] [PubMed] [Google Scholar]
- 68.Rothlin E. Pharmacology of lysergic acid diethylamide and some of its related compounds. J Pharm Pharmacol. 1957;9(9):569–87. doi: 10.1111/j.2042-7158.1957.tb12312.x. [DOI] [PubMed] [Google Scholar]
- 69.Ginzel KH, Mayer-Gross W. Prevention of psychological effects of d-lysergic acid diethylamide (LSD 25) by its 2-brom derivative (BOL 148). Nature. 1956;178(4526):210. doi: 10.1038/178210a0. [DOI] [PubMed] [Google Scholar]
- 70.Aghajanian GK, Foote WE, Sheard MH. Lysergic acid diethylamide: sensitive neuronal units in the midbrain raphe. Science. 1968;161:706–8. doi: 10.1126/science.161.3842.706. [DOI] [PubMed] [Google Scholar]
- 71.Aghajanian GK, Foote WE, Sheard MH. Action of psychotogenic drugs on single midbrain raphe neurons. J Pharmacol Exp Ther. 1970;171(2):178–87. [PubMed] [Google Scholar]
- 72.Aghajanian GK, Hailgler HJ. Hallucinogenic indoleamines: Preferential action upon presynaptic serotonin receptors. Psychopharmacology. 1975;1:619–29. [PubMed] [Google Scholar]
- 73.Rogawski MA, Aghajanian GK. Response of central monoaminergic neurons to lisuride: comparison with LSD. Life Sci. 1979;24(14):1289–97. doi: 10.1016/0024-3205(79)90148-6. [DOI] [PubMed] [Google Scholar]
- 74.Haigler HJ, Aghajanian GK. Mescaline and LSD: direct and indirect effects on serotonin-containing neurons in brain. Eur J Pharmacol. 1973;21(1):53–60. doi: 10.1016/0014-2999(73)90206-9. [DOI] [PubMed] [Google Scholar]
- 75.Trulson ME, Heym J, Jacobs BL. Dissociations between the effects of hallucinogenic drugs on behavior and raphe unit activity in freely moving cats. Brain Res. 1981;215(1−2):275–93. doi: 10.1016/0006-8993(81)90507-2. [DOI] [PubMed] [Google Scholar]
- 76.Colpaert FC, Niemegeers CJ, Janssen PA. A drug discrimination analysis of lysergic acid diethylamide (LSD): in vivo agonist and antagonist effects of purported 5-hydroxytryptamine antagonists and of pirenperone, a LSD-antagonist. J Pharmacol Exp Ther. 1982;221(1):206–14. [PubMed] [Google Scholar]
- 77.Sadzot B, Baraban JM, Glennon RA, Lyon RA, Leonhardt S, Jan CR, Titeler M. Hallucinogenic drug interactions at human brain 5-HT2 receptors: implications for treating LSD-induced hallucinogenesis. Psychopharmacology. 1989;98(4):495–9. doi: 10.1007/BF00441948. [DOI] [PubMed] [Google Scholar]
- 78.Sleight AJ, Stam NJ, Mutel V, Vanderheyden PML. Radiolabelling of the human 5-HT2A receptor with an agonist, a partial agonist and an antagonist: Effects on apparent agonist affinities. Biochemical Pharmacology. 1996;51(1):71–76. doi: 10.1016/0006-2952(95)02122-1. [DOI] [PubMed] [Google Scholar]
- 79.Aghajanian GK, Marek GJ. Serotonin and hallucinogens. Neuropsychopharmacology. 1999;21:16S–23S. doi: 10.1016/S0893-133X(98)00135-3. [DOI] [PubMed] [Google Scholar]
- 80.Marek GJ, Aghajanian GK. 5-HT2A receptor or alpha1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. Eur J Pharmacol. 1999;367(2−3):197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- 81.Zhou FM, Hablitz JJ. Activation of serotonin receptors modulates synaptic transmission in rat cerebral cortex. J Neurophysiol. 1999;82(6):2989–99. doi: 10.1152/jn.1999.82.6.2989. [DOI] [PubMed] [Google Scholar]
- 82.Lambe EK, Goldman-Rakic PS, Aghajanian GK. Serotonin induces EPSCs preferentially in layer V pyramidal neurons of the frontal cortex in the rat. Cereb Cortex. 2000;10(10):974–80. doi: 10.1093/cercor/10.10.974. [DOI] [PubMed] [Google Scholar]
- 83.Lambe EK, Aghajanian GK. The role of Kv1.2-containing potassium channels in serotonin-induced glutamate release from thalamocortical terminals in rat frontal cortex. J Neurosci. 2001;21(24):9955–63. doi: 10.1523/JNEUROSCI.21-24-09955.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beique JC, Chapin-Penick EM, Mladenovic L, Andrade R. Serotonergic facilitation of synaptic activity in the developing rat prefrontal cortex. J Physiol. 2004;556(Pt 3):739–54. doi: 10.1113/jphysiol.2003.051284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience. 2001;105(2):379–92. doi: 10.1016/s0306-4522(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 86.Béïque JC, Imad M, Mladenovic L, Gingrich JA, Andrade R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc Natl Acad Sci USA. 2007;104(23):9870–5. doi: 10.1073/pnas.0700436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lambe EK, Aghajanian GK. Prefrontal cortical network activity: Opposite effects of psychedelic hallucinogens and D1/D5 dopamine receptor activation. Neuroscience. 2007;145(3):900–10. doi: 10.1016/j.neuroscience.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nichols DE. Structure-activity relationships of phenethylamine hallucinogens. J Pharm Sci. 1981;70(8):839–49. doi: 10.1002/jps.2600700802. [DOI] [PubMed] [Google Scholar]
- 89.Glennon RA, McKenney JD, Lyon RA, Titeler M. 5-HT1 and 5-HT2 binding characteristics of 1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane analogues. J Med Chem. 1986;29(2):194–9. doi: 10.1021/jm00152a005. [DOI] [PubMed] [Google Scholar]
- 90.Glennon RA. Stimulus properties of hallucinogenic phenalkylamines and related designer drugs: formulation of structure-activity relationships. NIDA Res Monogr. 1989;94:43–67. [PubMed] [Google Scholar]
- 91.Glennon RA. Arylalkylamine drugs of abuse: an overview of drug discrimination studies. Pharmacol Biochem Behav. 1999;64(2):251–6. doi: 10.1016/s0091-3057(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 92.Nichols DE, Snyder SE, Oberlender R, Johnson MP, Huang XM. 2,3-Dihydrobenzofuran analogues of hallucinogenic phenethylamines. J Med Chem. 1991;34(1):276–81. doi: 10.1021/jm00105a043. [DOI] [PubMed] [Google Scholar]
- 93.Shulgin AT, Shulgin A. PIHKAL: a chemical love story. Transform Press; Berkley: 1991. [Google Scholar]
- 94.Jacob P, 3rd, Shulgin AT. Structure-activity relationships of the classic hallucinogens and their analogs. NIDA Res Monogr. 1994;146:74–91. [PubMed] [Google Scholar]
- 95.Nichols DE. Structure-activity relationships of phenethylamine hallucinogens. J Pharmaceutical Sci. 2006;70(8):839–49. doi: 10.1002/jps.2600700802. [DOI] [PubMed] [Google Scholar]
- 96.Sanders-Bush E, Burris KD, Knoth K. Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis. J Pharmacol Exp Ther. 1988;246(3):924–8. [PubMed] [Google Scholar]
- 97.Sanders-Bush E, Breeding M. Choroid plexus epithelial cells in primary culture: a model of 5HT1C receptor activation by hallucinogenic drugs. Psychopharmacology. 1991;105(3):340–6. doi: 10.1007/BF02244428. [DOI] [PubMed] [Google Scholar]
- 98.Winter JC. Stimulus properties of phenethylamine hallucinogens and lysergic acid diethylamide: the role of 5-hydroxytryptamine. J Pharmacol Exp Ther. 1978;204(2):416–23. [PubMed] [Google Scholar]
- 99.Glennon RA, Rosecrans JA, Young R. Drug-induced discrimination: a description of the paradigm and a review of its specific application to the study of hallucinogenic agents. Med Res Rev. 1983;3(3):289–340. doi: 10.1002/med.2610030305. [DOI] [PubMed] [Google Scholar]
- 100.Fiorella D, Rabin RA, Winter JC. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. I: Antagonist correlation analysis. Psychopharmacology. 1995;121(3):347–56. doi: 10.1007/BF02246074. [DOI] [PubMed] [Google Scholar]
- 101.Schreiber R, Brocco M, Millan MJ. Blockade of the discriminative stimulus effect of DOI by MDL 100,907 and the “atypical” antipsychotics, clozapine and risperidone. Eu J Pharmacol. 1994;264:99–102. doi: 10.1016/0014-2999(94)90643-2. [DOI] [PubMed] [Google Scholar]
- 102.Gonzalez-Maeso J, Yuen T, Ebersole BJ, Wurmbach E, Lira A, Zhou M, Weisstaub N, Hen R, Gingrich JA, Sealfon SC. Transcriptome fingerprints distinguish hallucinogenic and nonhallucinogenic 5-hydroxytryptamine 2A receptor agonist effects in mouse somatosensory cortex. J Neurosci. 2003;23(26):8836–43. doi: 10.1523/JNEUROSCI.23-26-08836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Torda T, Culman J, Cechova E, Murgas K. 3-H-ketanserin (serotonin type 2) binding in the rat frontal cortex: effect of immobilization stress. Endocrinol Exp. 1988;22(2):99–105. [PubMed] [Google Scholar]
- 104.McKittrick CR, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Serotonin receptor binding in a colony model of chronic social stress. Biol Psychiatry. 1995;37(6):383–93. doi: 10.1016/0006-3223(94)00152-s. [DOI] [PubMed] [Google Scholar]
- 105.Takao K, Nagatani T, Kitamura Y, Kawasaki K, Hayakawa H, Yamawaki S. Chronic forced swim stress of rats increases frontal cortical 5-HT2 receptors and the wet-dog shakes they mediate, but not frontal cortical beta-adrenoceptors. Eur J Pharmacol. 1995;294(2−3):721–6. doi: 10.1016/0014-2999(95)00620-6. [DOI] [PubMed] [Google Scholar]
- 106.Berton O, Aguerre S, Sarrieau A, Mormede P, Chaouloff F. Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience. 1998;82(1):147–59. doi: 10.1016/s0306-4522(97)00282-0. [DOI] [PubMed] [Google Scholar]
- 107.Ossowska G, Nowa G, Kata R, Klenk-Majewska B, Danilczuk Z, Zebrowska-Lupina I. Brain monoamine receptors in a chronic unpredictable stress model in rats. J Neural Transm. 108(3):311–9. doi: 10.1007/s007020170077. [DOI] [PubMed] [Google Scholar]
- 108.Goodwin GM, Green AR, Johnson P. 5-HT2 receptor characteristics in frontal cortex and 5-HT2 receptor-mediated head-twitch behaviour following antidepressant treatment to mice. Br J Pharmacol. 1984;83(1):235–42. doi: 10.1111/j.1476-5381.1984.tb10140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Metz A, Heal DJ. In mice repeated administration of electroconvulsive shock or desmethylimipramine produces rapid alterations in 5-HT2-mediated head-twitch responses and cortical 5-HT2 receptor number. Eur J Pharmacol. 1986;126(1−2):159–62. doi: 10.1016/0014-2999(86)90754-5. [DOI] [PubMed] [Google Scholar]
- 110.Isbell H, Miner EJ, Logan CR. Cross tolerance between D-2-brom-lysergic acid diethylamide (BOL-148) and the D-diethylamide of lysergic acid (LSD-25). Psychopharmacologia. 1959;1:109–16. doi: 10.1007/BF00409110. [DOI] [PubMed] [Google Scholar]
- 111.Leysen JE, Janssen PF, Niemegeers CJ. Rapid desensitization and down-regulation of 5-HT2 receptors by DOM treatment. Eur J Pharmacol. 1989;163(1):145–9. doi: 10.1016/0014-2999(89)90409-3. [DOI] [PubMed] [Google Scholar]
- 112.Schlemmer RF, Jr, Davis JM. A primate model for the study of hallucinogens. Pharmacol Biochem Behav. 1986;24(2):381–92. doi: 10.1016/0091-3057(86)90368-0. [DOI] [PubMed] [Google Scholar]
- 113.Buckholtz NS, Freedman DX, Middaugh LD. Daily LSD administration selectively decreases serotonin2 receptor binding in rat brain. Eur J Pharmacol. 1985;109(3):421–5. doi: 10.1016/0014-2999(85)90407-8. [DOI] [PubMed] [Google Scholar]
- 114.McKenna DJ, Nazarali AJ, Himeno A, Saavedra JM. Chronic treatment with (+/−)DOI, a psychotomimetic 5-HT2 agonist, downregulates 5-HT2 receptors in rat brain. Neuropsychopharmacology. 1989;2(1):81–7. doi: 10.1016/0893-133x(89)90010-9. [DOI] [PubMed] [Google Scholar]
- 115.Owens MJ, Knight DL, Ritchie JC, Nemeroff CB. The 5-hydroxytryptamine2 agonist, (+−)-1-(2,5-dimethoxy-4-bromophenyl)-2-aminopropane stimulates the hypothalamic-pituitary-adrenal (HPA) axis. II. Biochemical and physiological evidence for the development of tolerance after chronic administration. J Pharmacol Exp Ther. 1991;256(2):795–800. [PubMed] [Google Scholar]
- 116.Berendsen HH, Broekkamp CL. Attenuation of 5-HT1A and 5-HT2 but not 5-HT1C receptor mediated behaviour in rats following chronic treatment with 5-HT receptor agonists, antagonists or anti-depressants. Psychopharmacology. 1991;105(2):219–24. doi: 10.1007/BF02244313. [DOI] [PubMed] [Google Scholar]
- 117.Smith RL, Barrett RJ, Sanders-Bush E. Mechanism of tolerance development to 2,5-dimethoxy-4-iodoamphetamine in rats: down-regulation of the 5-HT2A, but not 5-HT2C, receptor. Psychopharmacology. 1999;144(3):248–54. doi: 10.1007/s002130051000. [DOI] [PubMed] [Google Scholar]
- 118.Shulgin AT, Shulgin A. TIHKAL: the continuation. Transform Press; Berkley: 1997. [Google Scholar]
- 119.Schultes RE. Hallucinogenic plants: their earliest botanical descriptions. J Psychedelic Drugs. 1979;11(1−2):13–24. doi: 10.1080/02791072.1979.10472087. [DOI] [PubMed] [Google Scholar]
- 120.Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53(3):439–52. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 121.Sprouse JS, Aghajanian GK. Electrophysiological responses of serotoninergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1(1):3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- 122.Sprouse JS, Aghajanian GK. Responses of hippocampal pyramidal cells to putative serotonin 5-HT1A and 5-HT1B agonists: a comparative study with dorsal raphe neurons. Neuropharmacology. 1988;27(7):707–15. doi: 10.1016/0028-3908(88)90079-2. [DOI] [PubMed] [Google Scholar]
- 123.Spencer DG, Jr, Glaser T, Traber J. Serotonin receptor subtype mediation of the interoceptive discriminative stimuli induced by 5-methoxy-N,N-dimethyltryptamine. Psychopharmacology. 1987;93(2):158–66. doi: 10.1007/BF00179927. [DOI] [PubMed] [Google Scholar]
- 124.Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: an indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacology Biochemistry and Behavior. 2000;65(1):75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- 125.Reissig CJ, Eckler JR, Rabin RA, Winter JC. The 5-HT1A receptor and the stimulus effects of LSD in the rat. Psychopharmacology. 2005;182(2):197–204. doi: 10.1007/s00213-005-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. British Journal of Pharmacology. 1999;128(1):13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bunzow JR, Sonders MS, Arttamangkul S, Harrison LM, Zhang G, Quigley DI, Darland T, Suchland KL, Pasumamula S, Kennedy JL, Olson SB, Magenis RE, Amara SG, Grandy DK. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001;60(6):1181–8. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- 128.Premont RT, Gainetdinov RR, Caron MG. Following the trace of elusive amines. Proc Natl Acad Sci USA. 2001;98:9474–9475. doi: 10.1073/pnas.181356198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jacob MS, Presti DE. Endogenous psychoactive tryptamines reconsidered: an anxiolytic role for dimethyltryptamine. Med Hypotheses. 2005;64(5):930–7. doi: 10.1016/j.mehy.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 130.Lewin AH. Receptors of mammalian trace amines. AAPS J. 2006;8(1):E138–45. doi: 10.1208/aapsj080116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abraham HD, Aldridge AM, Gogia P. The psychopharmacology of hallucinogens. Neuropsychopharmacology. 1996;14:285–98. doi: 10.1016/0893-133X(95)00136-2. [DOI] [PubMed] [Google Scholar]
- 132.Halpern JH, Pope HG., Jr Hallucinogen persisting perception disorder: what do we know after 50 years? Drug Alcohol Depend. 2003;69:109–19. doi: 10.1016/s0376-8716(02)00306-x. [DOI] [PubMed] [Google Scholar]
- 133.American Psychiatric Association., American Psychiatric Association . Diagnostic and statistical manual of mental disorders : DSM-IV. American Psychiatric Association; Washington, DC: 1994. Task Force on DSM-IV. [Google Scholar]
- 134.Moskowitz D. Use of haloperidol to reduce LSD flashbacks. Military Medicine. 1971;136:754–6. [PubMed] [Google Scholar]
- 135.Glennon RA, Titeler M, McKenney JD. Evidence for 5-HT2 involvement in the mechanism of action of hallucinogenic agents. Life Sci. 1984;35:2505–11. doi: 10.1016/0024-3205(84)90436-3. [DOI] [PubMed] [Google Scholar]
- 136.Glennon RA, Young R, Rosecrans JA. Antagonism of the effects of the hallucinogen DOM and the purported 5-HT agonist quipazine by 5-HT2 antagonists. Eur J Pharmacol. 1983;91:189–96. doi: 10.1016/0014-2999(83)90464-8. [DOI] [PubMed] [Google Scholar]
- 137.Pazos A, Castro ME, del Olmo E, Romon T, del Arco C. Autoradiographic characterization, anatomical distribution, and developmental pattern of a new 5-HT site in human brain. Ann N Y Acad Sci. 1998;861:262. doi: 10.1111/j.1749-6632.1998.tb10213.x. [DOI] [PubMed] [Google Scholar]
- 138.Pazos A, Cortes R, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985;346:231–49. doi: 10.1016/0006-8993(85)90857-1. [DOI] [PubMed] [Google Scholar]
- 139.Sorensen SM, Kehne JH, Fadayel GM, Humphreys TM, Ketteler HJ, Sullivan CK, et al. Characterization of the 5-HT2 receptor antagonist MDL 100907 as a putative atypical antipsychotic: behavioral, electrophysiological and neurochemical studies. J Pharmacol Exp Ther. 1993;266:684–91. [PubMed] [Google Scholar]
- 140.Gresch PJ, Barrett RJ, Sanders-Bush E, Smith RL. 5-Hydroxytryptamine (serotonin)2A receptors in rat anterior cingulate cortex mediate the discriminative stimulus properties of d-lysergic acid diethylamide. J Pharmacol Exp Ther. 2007;320:662–9. doi: 10.1124/jpet.106.112946. [DOI] [PubMed] [Google Scholar]
- 141.Molliver ME. Serotonergic neuronal systems: what their anatomic organization tells us about function. J Clin Psychopharmacol. 1987;7:3S–23S. [PubMed] [Google Scholar]
- 142.Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–30. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- 143.Arvanov VL, Liang X, Russo A, Wang RY. LSD and DOB: interaction with 5-HT2A receptors to inhibit NMDA receptor-mediated transmission in the rat prefrontal cortex. Eur J Neurosci. 1999;11:3064–72. doi: 10.1046/j.1460-9568.1999.00726.x. [DOI] [PubMed] [Google Scholar]
- 144.Gewirtz JC, Marek GJ. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology. 2000;23:569–76. doi: 10.1016/S0893-133X(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 145.Meltzer HY. The role of serotonin in antipsychotic drug action. Neuropsychopharmacology. 1999;21:106S–15S. doi: 10.1016/S0893-133X(99)00046-9. [DOI] [PubMed] [Google Scholar]
- 146.Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–72. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 147.Nielsen EB. Discriminative stimulus properties of lysergic acid diethylamide in the monkey. J Pharmacol Exp Ther. 1985;234(1):244–9. [PubMed] [Google Scholar]
- 148.Appel JB, West WB, Rolandi WG, Alici T, Pechersky K. Increasing the selectivity of drug discrimination procedures. Pharmacol Biochem Behav. 1999;64(2):353–8. doi: 10.1016/s0091-3057(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 149.Callahan PM, Appel JB. Differentiation between the stimulus effects of (+)-lysergic acid diethylamide and lisuride using a three-choice, drug discrimination procedure. Psychopharmacology. 1990;100(1):13–8. doi: 10.1007/BF02245782. [DOI] [PubMed] [Google Scholar]
- 150.Lees AJ, Bannister R. The use of lisuride in the treatment of multiple system atrophy with autonomic failure (Shy-Drager syndrome). J Neurol Neurosurg Psychiatry. 1981;44:347–351. doi: 10.1136/jnnp.44.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Parkes JD, Schachter M, Marsden CD, Smith B, Wilson A. Lisuride in parkinsonism. Ann Neurol. 1981;9(1):48–52. doi: 10.1002/ana.410090109. [DOI] [PubMed] [Google Scholar]
- 152.Lieberman A, Goldstein M, Neophytides A, Kupersmith M, Leibowitz M, Zasorin N, Walker R, Kleinberg D. Lisuride in Parkinson disease: efficacy of lisuride compared to levodopa. Neurology. 1981;31(8):961–965. doi: 10.1212/wnl.31.8.961. [DOI] [PubMed] [Google Scholar]
- 153.Gopinathan G, Teravainen H, Dambrosia JM, Ward CD, Sanes JN, Stuart WK, Evarts EV, Calne DB. Lisuride in parkinsonism. Neurology. 1981;31(4):371–376. doi: 10.1212/wnl.31.4.371. [DOI] [PubMed] [Google Scholar]
- 154.Critchley P, Grandas Perez F, Quinn N, Coleman R, Parkes D, Marsden CD. Psychosis and the lisuride pump. Lancet. 1986;2(8502):349. doi: 10.1016/s0140-6736(86)90042-5. [DOI] [PubMed] [Google Scholar]
- 155.Todes CJ. At the receiving end of the lisuride pump. Lancet. 1986;2(8497):36–37. doi: 10.1016/s0140-6736(86)92576-6. [DOI] [PubMed] [Google Scholar]
- 156.Horowski R. Psychiatric side-effects of high-dose lisuride therapy in parkinsonism. Lancet. 1986;2(8505):510. doi: 10.1016/s0140-6736(86)90374-0. [DOI] [PubMed] [Google Scholar]
- 157.Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, et al. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacology & therapeutics. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- 158.Rabin RA, Regina M, Doat M, Winter JC. 5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens. Pharmacol Biochem Behav. 2002;72:29–37. doi: 10.1016/s0091-3057(01)00720-1. [DOI] [PubMed] [Google Scholar]
- 159.Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther. 2003;304:229–37. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- 160.Berg KA, Maayani S, Goldfarb J, Scaramellini C, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Molecular Pharmacology. 1998;54:94–104. [PubMed] [Google Scholar]
- 161.Cox DA, Watts SW, Cohen ML. Neomycin selectively inhibits 5-hydroxytryptamine-induced contraction in the guinea pig trachea. J Pharmacol Exp Ther. 1996;277:954–9. [PubMed] [Google Scholar]
- 162.Berg KA, Maayani S, Goldfarb J, Scaramellini L, Leff P, Clarke WP. Effector pathway-dependent relative efficacy at serotonin type 2A and 2C receptors: evidence for agonist-directed trafficking of receptor stimulus. Mol Pharmacol. 1998;54:94–104. [PubMed] [Google Scholar]
- 163.Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, Kobilka BK. Functionally different agonists induce distinct conformations in the G protein coupling domain of the beta 2 adrenergic receptor. J Biol Chem. 2001;276:24433–24436. doi: 10.1074/jbc.C100162200. [DOI] [PubMed] [Google Scholar]
- 164.Nair VD, Sealfon SC. Agonist specific transactivation of phosphoinositide 3-kinase signaling pathway mediated by the dopamine D2 receptor. J Biol Chem. 2003;47:47053–47061. doi: 10.1074/jbc.M303364200. [DOI] [PubMed] [Google Scholar]
- 165.Kenakin T. Ligand-selective receptor conformations revisited: the promise and the problem. Trends Pharmacol Sci. 2003;24:346–354. doi: 10.1016/S0165-6147(03)00167-6. [DOI] [PubMed] [Google Scholar]
- 166.Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka BK, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton P, et al. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2006;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- 167.McKenna DJ, Peroutka SJ. Differentiation of 5-hydroxytryptamine2 receptor subtypes using 125I-R-(−)2,5-dimethoxy-4-iodo-phenylisopropylamine and 3H-ketanserin. J Neurosci. 1989;9(10):3482–90. doi: 10.1523/JNEUROSCI.09-10-03482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.May JA, Chen HH, Rusinko A, Lynch VM, Sharif NA, McLaughlin MA. A novel and selective 5-HT2 receptor agonist with ocular hypotensive activity: (S)-(+)-1-(2-aminopropyl)-8,9-dihydropyrano[3,2-e]indole. J Med Chem. 2003;46(19):4188–95. doi: 10.1021/jm030205t. [DOI] [PubMed] [Google Scholar]
- 169.Pauwels PJ, Van Gompel P, Leysen JE. Activity of serotonin (5-HT) receptor agonists, partial agonists and antagonists at cloned human 5-HT1A receptors that are negatively coupled to adenylate cyclase in permanently transfected HeLa cells. Biochem Pharmacol. 1993;45(2):375–83. doi: 10.1016/0006-2952(93)90073-6. [DOI] [PubMed] [Google Scholar]
- 170.Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT(2A) and 5-HT(2C) receptors. Synapse. 2000;35(2):144–50. doi: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 171.Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33(3−4):275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 172.Titeler M, Lyon RA, Glennon RA. Radioligand binding evidence implicates the brain 5-HT2 receptor as a site of action for LSD and phenylisopropylamine hallucinogens. Psychopharmacology. 1988;94(2):213–6. doi: 10.1007/BF00176847. [DOI] [PubMed] [Google Scholar]
- 173.Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch Pharmacol. 2004;370(2):114–23. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- 174.Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Cumbay MG, Watts VJ, Barker EL, Nichols DE. Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J Med Chem. 2000;43(24):4701–10. doi: 10.1021/jm000339w. [DOI] [PubMed] [Google Scholar]
- 175.Nichols DE, Frescas S, Marona-Lewicka D, Kurrasch-Orbaugh DM. Lysergamides of isomeric 2,4-dimethylazetidines map the binding orientation of the diethylamide moiety in the potent hallucinogenic agent N,N-diethyllysergamide (LSD). J Med Chem. 2002;45(19):4344–9. doi: 10.1021/jm020153s. [DOI] [PubMed] [Google Scholar]
- 176.Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A. Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes. J Pharmacol Exp Ther. 2002;303(2):791–804. doi: 10.1124/jpet.102.039867. [DOI] [PubMed] [Google Scholar]
- 177.Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT(2A) and 5-HT(2C) receptors. Synapse. 2000;35(2):144–50. doi: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 178.Pierce PA, Peroutka SJ. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology. 1989;97(1):118–22. doi: 10.1007/BF00443425. [DOI] [PubMed] [Google Scholar]