One of the crucial events in eukaryotic gene regulation is the assembly of general transcription factors and RNA polymerase II (Pol II) into a pre-initiation complex (PIC) at promoter regions. The textbook description of mRNA synthesis initiation requires the recruitment and binding of the TATA-binding protein (TBP)—a component of the TFIID complex—to the TATA box (Butler & Kadonaga, 2002). Several studies are now changing this idea by showing that TBP-independent Pol II transcription can occur (Paulson et al, 2002; Wieczorek et al, 1998), and that other types of TBP-related factors (TRFs) can mediate Pol II transcription of development regulator genes in vertebrate embryos in the absence of TBP (Martianov et al, 2002; Müller et al, 2001; Veenstra et al, 2000). In addition to TBP, two other TRFs have been characterized in vertebrates and were suggested to function in Pol II transcription initiation: TBP-like factor (TLF, also called TRF2, TLP or TBPL1) and TBP2 (also called TRF3 or TBPL2; Bartfai et al, 2004; Dantonel et al, 1999; Jallow et al, 2004; Persengiev et al, 2003, and references therein).
Given the diversity of TBP-related proteins (Fig 1), several important questions arise. Is there any functional specialization for TBP and TBP-related factors in vivo? Do the requirements of these factors for transcriptional activation vary according to the groups of genes involved in specific cellular processes? One direct way to address these questions is to investigate the role of these factors during early development, as distinct groups of ‘developmental' genes can then be analysed. Furthermore, the embryo switches from a genomic ‘transcription off' to a ‘transcription on' state, which in Xenopus and zebrafish occurs during the midblastula transition (MBT).
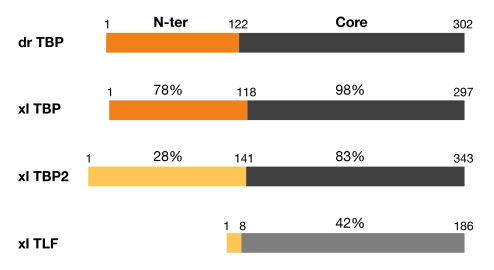
Figure 1.
Schematic representation and domain comparison of TATA-binding protein-related factors. TBP from zebrafish (Danio rerio; dr) and three TBP-related proteins from Xenopus laevis (xl) are shown. Numbers indicate amino-acid positions. The identity between the amino-terminal (N-ter) and core domains is indicated in percentage when compared with the corresponding domain of xlTBP. TBP, TATA-binding protein; TLF, TBP-like factor.
In the 5 September 2007 issue of The EMBO Journal, the F. Müller and G.J. Veenstra laboratories reported the characterization of the specific TRF requirements for gene expression in zebrafish and Xenopus. Both compared genome-wide gene expression profiles immediately after the MBT in control embryos and in embryos in which TBP or TBP-related factors had been knocked down (Ferg et al, 2007; Jacobi et al, 2007).
The Veenstra laboratory performed TBP, TLF or TBP2 depletion and focused on the analysis of newly transcribed genes in X. leavis and X. tropicalis (Jacobi et al, 2007). Transcripts that are present in the embryo before MBT are maternal in origin; therefore, the authors distinguished between zygotic and maternal genes by comparing the microarray data with controls at the blastula stage—that is, before MBT. Although overexpression of TBP2 has been shown previously to partly rescue TBP deficiency in embryos (Jallow et al, 2004), single knockdowns of each of the three factors showed a clear preference for target genes. The strongest effect on gene expression was observed when either TLF or TBP2 were silenced—74% and 60% of genes were affected, respectively. By contrast, 69% of the expressed genes were unaffected when TBP was knocked down. This result is similar to the findings in zebrafish reported by Ferg et al (2007), who documented that among approximately 2,000 genes selected, 65% seemed to be TBP-independent. These surprising new observations suggest that—contrary to the textbook dogma—TBP is required at these early stages of development for the transcription of only one-third of the total transcribed genes, and also that TBP2 and TLF have more important roles in Pol II transcription than previously anticipated. In Xenopus, TBP did not seem to be required for transcription of newly transcribed genes that were not present maternally. Jacobi et al (2007) conclude that TBP-dependent genes are ‘non-specialized' and are expressed either in the adult or maternally.
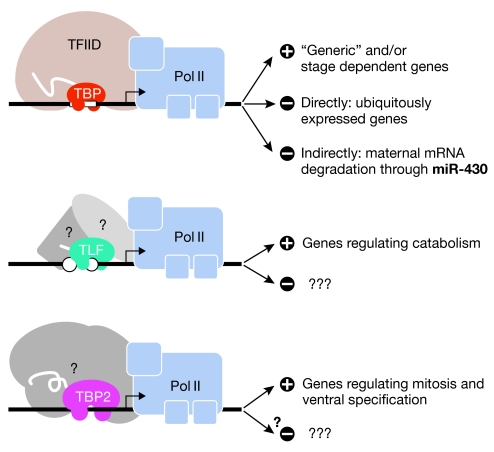
Jacobi et al (2007) explored a possible allocation of the genes regulated by TBP, TBP2 or TLF into different ontology groups, with the aim of identifying the requirements of these factors in specific cellular processes. Important physiological processes seem to be regulated by the three factors; however, several essential cellular processes are regulated by more than one factor. In addition, TLF seemed to be required for genes that are preferentially expressed in the embryo and TBP2 for genes involved in ventral specification during development (Fig 2). This is in agreement with the demonstration that TBP2 knockdown leads to dorsalization in zebrafish (Bartfai et al, 2004). Chromatin immunoprecipitation (ChIP) assays were used to establish a link between binding by TBP or TBP2 and regulation of the corresponding genes. In most cases, an exclusive requirement for TBP or TBP2 reflected a direct, exclusive recruitment of either factor onto a promoter. However, some promoters recruited both factors equally well, which could reflect a possible functional cross-talk between the two factors on some genes; however, ChIP assays cannot eliminate the possibility of a given promoter being occupied by different factors in different cell types. In any case, one can imagine that only a well-defined TBP-related factor would allow correct levels of transcription initiation and that the presence of one of the others would misregulate the expression of a given gene. In agreement with this model, the depletion of TLF seems to upregulate TBP2-dependent genes (Jacobi et al, 2007). Interestingly, TBP and/or TBP2 occupancy in Xenopus did not necessarily correlate with transcription, which agrees with the fact that RNA Pol II recruitment to a promoter does not always correlate with active transcription (Lee et al, 2006). Future studies should determine how the recruitment of each of these three factors might respond to local changes of chromatin structure. Do these changes vary according to the different TBP-related factors and their regulated promoters? Are they determined by specific chromatin modifiers during a particular developmental time window?
Figure 2.
Types of genes regulated by TATA-binding protein-related factors. The three factors (TBP, TLF and TBP2) regulate transcription of different target genes, and TBP also regulates maternal mRNA degradation through miR-430. TBP, TLF and TBP2 binding to different promoters are depicted. The hypothetical TLF- or TBP2-associated factors are shown in grey. The gene categories described by Ferg et al (2007) and Jacobi et al (2007), which are regulated by one of the three TBP-related factors are summarized on the right; none of these categories are exclusive. Question marks indicate that this activity has not been reported. TBP, TATA-binding protein; TLF, TBP-like factor.
Using the zebrafish as a model, F. Müller's laboratory analysed the requirement for TBP during MBT by combining TBP knockdown and microarray profiling (Ferg et al, 2007). As mentioned above, a large majority of genes turned out to be TBP-independent. The authors then focused on the genes with altered expression in TBP knock down embryos, and found that 17.5% were down regulated and 17.5% upregulated. Developmentally regulated—that is, stage-dependent—genes were overrepresented among those that showed a TBP requirement for their activation (Fig 2), which is in agreement with previous observations in mammals (Bajic et al, 2006; Sandelin et al, 2007). By contrast, constitutively expressed genes were more likely to be negatively regulated by TBP. Ferg et al (2007) studied whether TBP directly influences the transcription of genes expressed immediately after MBT. Out of 12 promoters that showed a change in activity in the absence of TBP, transcription from 7 decreased and transcription from 4 increased. This suggests that TBP acts as a direct repressor of transcription, although further experiments are needed to determine how TBP carries out this repressive function.
Genome activation and degradation of the maternal mRNAs are essential processes for the maternal-to-zygotic transition. A long-standing question in developmental biology is whether there is any cross-talk between these two processes and, if so, what are the molecular mechanisms involved. The Müller laboratory explored whether TBP could modulate the mRNA degradation of the genes upregulated upon TBP knockdown. They found that maternal mRNAs were significantly enriched in genes upregulated in TBP knockdown embryos, and that this abnormal persistence resulted from a lack of maternal mRNA degradation and not a premature activation of zygotic mRNAs. MicroRNAs (miRNAs)—miR-430 in particular—have recently been shown to have a direct role in the degradation of many maternal mRNAs through regulation of polyadenylation in zebrafish (Giraldez et al, 2006). In addition, the maternal deletion of Dicer in mice results in the inability of the embryos to develop beyond the zygote stage and in an abnormal accumulation of maternal mRNAs (Tang et al, 2007). A striking observation made by Ferg et al (2007) is that the set of genes in which degradation is dependent on miR-430 is overrepresented in TBP knockdown embryos. To test the possibility of a link between the miR-430 pathway and TBP, the authors injected synthetic mRNAs with untranslated regions containing miR-430 target sequences at the zygote stage. These chimaeric mRNAs were degraded after MBT in control embryos, but not in TBP knockdown embryos, whereas the degradation of mRNAs containing target sequences for an unrelated miRNA was unaffected. Furthermore, overexpression of TBP resulted in increased degradation of specific mRNAs. Expression of miR-430 was not affected in TBP morphants, which suggests that TBP acts downstream from miR-430 to regulate mRNA degradation. Could TBP be regulating the assembly of the RNA-induced silencing complex (RISC) through indirect mechanisms? Is there a direct interaction between TBP and the RNAi machinery? Argonaute proteins have been shown to immunoprecipitate with the RNA Pol II (Kim et al, 2006); therefore, the possibility of a direct and perhaps regulatory interaction between RISC and TBP is plausible.
The findings reported by the laboratories of G.J. Veenstra and F. Müller raise interesting evolutionary perspectives and an obvious question: what happens in mammals? This will probably be a complex answer, particularly because the developmental stage at which genome activation occurs varies considerably between species: it occurs at the 1–2-cell stage in mice, the 4–8-cell stage in cows and humans, and the 8–16-cell stage in sheep and rabbits (Schultz & Heyner, 1992). Similarly to zebrafish, TBP protein levels increase in the mouse embryo around zygotic genome activation (Gazdag et al, 2007) and mouse embryos in which zygotic TBP contribution has been depleted do exhibit some levels, albeit reduced, of Pol II transcription (Martianov et al, 2002). Recent reports suggest that, in the mouse, TBP2 has a more important role during oogenesis than TBP (Gazdag et al, 2007; Xiao et al, 2006), similar to TLF during spermatogenesis (Martianov et al, 2001).
The observations of Ferg et al (2007) and Jacobi et al (2007) nicely illustrate that general transcription factors—such as TBP-related factors—can act as specific regulators of gene expression during development (Fig 2). Most importantly, they have uncovered an unexpected role for TBP in the degradation of a subset of maternal mRNAs through the miRNA pathway, placing TBP as an important component of the maternal-to-zygotic transition in the vertebrate embryo.
References
- Bajic VB et al. (2006) Mice and men: their promoter properties. PLoS Genet 2: e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfai R, Balduf C, Hilton T, Rathmann Y, Hadzhiev Y, Tora L, Orban L, Muller F (2004) TBP2, a vertebrate-specific member of the TBP family, is required in embryonic development of zebrafish. Curr Biol 14: 593–598 [DOI] [PubMed] [Google Scholar]
- Butler JE, Kadonaga JT (2002) The RNA polymerase II core promoter: a key component in the regulation of gene expression. Genes Dev 16: 2583–2592 [DOI] [PubMed] [Google Scholar]
- Dantonel JC, Wurtz JM, Poch O, Moras D, Tora L (1999) The TBP-like factor: an alternative transcription factor in metazoa? Trends Biochem Sci 24: 335–339 [DOI] [PubMed] [Google Scholar]
- Ferg M et al. (2007) The TATA-binding protein regulates maternal mRNA degradation and differential zygotic transcription in zebrafish. EMBO J 26: 3945–3956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazdag E, Rajkovic A, Torres-Padilla ME, Tora L (2007) Analysis of TATA-binding protein 2 (TBP2) and TBP expression suggests different roles for the two proteins in regulation of gene expression during oogenesis and early mouse development. Reproduction 134: 51–62 [DOI] [PubMed] [Google Scholar]
- Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, Enright AJ, Schier AF (2006) Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science 312: 75–79 [DOI] [PubMed] [Google Scholar]
- Jacobi UG, Akkers RC, Pierson ES, Weeks DL, Dagle JM, Veenstra GJ (2007) TBP paralogs accommodate metazoan- and vertebrate-specific developmental gene regulation. EMBO J 26: 3900–3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jallow Z, Jacobi UG, Weeks DL, Dawid IB, Veenstra GJ (2004) Specialized and redundant roles of TBP and a vertebrate-specific TBP paralog in embryonic gene regulation in Xenopus. Proc Natl Acad Sci USA 101: 13525–13530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Villeneuve LM, Morris KV, Rossi JJ (2006) Argonaute-1 directs siRNA-mediated transcriptional gene silencing in human cells. Nat Struct Mol Biol 13: 793–797 [DOI] [PubMed] [Google Scholar]
- Lee TI et al. (2006) Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 125: 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martianov I, Fimia GM, Dierich A, Parvinen M, Sassone-Corsi P, Davidson I (2001) Late arrest of spermiogenesis and germ cell apoptosis in mice lacking the TBP-like TLF/TRF2 gene. Mol Cell 7: 509–515 [DOI] [PubMed] [Google Scholar]
- Martianov I, Viville S, Davidson I (2002) RNA polymerase II transcription in murine cells lacking the TATA binding protein. Science 298: 1036–1039 [DOI] [PubMed] [Google Scholar]
- Müller F, Lakatos L, Dantonel J, Strahle U, Tora L (2001) TBP is not universally required for zygotic RNA polymerase II transcription in zebrafish. Curr Biol 11: 282–287 [DOI] [PubMed] [Google Scholar]
- Paulson M, Press C, Smith E, Tanese N, Levy DE (2002) IFN-Stimulated transcription through a TBP-free acetyltransferase complex escapes viral shutoff. Nat Cell Biol 4: 140–147 [DOI] [PubMed] [Google Scholar]
- Persengiev SP, Zhu X, Dixit BL, Maston GA, Kittler EL, Green MR (2003) TRF3, a TATA-box-binding protein-related factor, is vertebrate-specific and widely expressed. Proc Natl Acad Sci USA 100: 14887–14891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelin A, Carninci P, Lenhard B, Ponjavic J, Hayashizaki Y, Hume DA (2007) Mammalian RNA polymerase II core promoters: insights from genome-wide studies. Nat Rev Genet 8: 424–436 [DOI] [PubMed] [Google Scholar]
- Schultz GA, Heyner S (1992) Gene expression in pre-implantation mammalian embryos. Mutat Res 296: 17–31 [DOI] [PubMed] [Google Scholar]
- Tang F, Kaneda M, O'Carroll D, Hajkova P, Barton SC, Sun YA, Lee C, Tarakhovsky A, Lao K, Surani MA (2007) Maternal microRNAs are essential for mouse zygotic development. Genes Dev 21: 644–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra GJ, Weeks DL, Wolffe AP (2000) Distinct roles for TBP and TBP-like factor in early embryonic gene transcription in Xenopus. Science 290: 2312–2315 [DOI] [PubMed] [Google Scholar]
- Wieczorek E, Brand M, Jacq X, Tora L (1998) Function of TAF(II)-containing complex without TBP in transcription by RNA polymerase II. Nature 393: 187–191 [DOI] [PubMed] [Google Scholar]
- Xiao L, Kim M, DeJong J (2006) Developmental and cell type-specific regulation of core promoter transcription factors in germ cells of frogs and mice. Gene Expr Patterns 6: 409–419 [DOI] [PubMed] [Google Scholar]