Abstract
Tumours develop in vertebrate organisms endowed with immune systems that are potentially able to eradicate them. Nevertheless, our ever-increasing understanding of the complex interactions between lymphocytes and tumour cells fuels the long-standing hope of developing efficient immunotherapies against cancer. This review focuses on a versatile family of proteins, the major histocompatibility complex class Ib, which has been recently implicated in both the establishment of anti-tumour immune responses and in tumour immune response evasion. We focus on a subset of class Ib proteins, human leukocyte antigen (HLA)-G, Qa-2, CD1d and NKG2D ligands, which bind to either stimulatory or inhibitory receptors expressed on T, natural killer (NK) and NKT lymphocytes, and thereby modulate their anti-tumour activity.
Keywords: MHC, cancer, innate, lymphocyte, natural killer
Introduction
Paul Ehrlich's discoveries 100 years ago seduced immunologists with the idea that cytotoxic cells are able to eliminate tumours, much like they destroy virus-infected cells. In the 1950s, MacFarlane Burnet proposed that the immune system surveys cellular transformation and prevents the development of tumours (Burnet, 1957). This ‘tumour surveillance hypothesis' has prompted the identification of a panoply of tumour-specific or tumour-enriched peptides over the past 20 years. These peptides are presumably the antigens presented by classical major histocompatibility complex (MHC) molecules to the cytotoxic T lymphocytes (CTL) of the adaptive immune system, particularly T-cell receptor (TCR) αβ+ CD8+ cells. Although their identification allowed the development of tumour vaccines—which are being assayed in clinical trials—the overall success of such immunotherapeutic strategies has been limited by the common downregulation of such antigens and/or their MHC class Ia presentation elements in advanced tumours. However, cancer immune surveillance is likely to involve both the adaptive and the innate immune systems, as is true for host immunity to pathogens. Indeed, many reports have illustrated the capacity of innate lymphocytes, γδ T, natural killer (NK) and NKT cells, to detect and destroy tumour cells independently of classical MHC presentation (Girardi et al, 2001, 2003; Smyth et al, 2001; Street et al, 2004).
The molecular mechanisms responsible for the immune recognition of tumour cells are still the subject of much debate. However, recent data has highlighted the many roles played by members of a plastic protein family: the non-classical or class Ib MHC (MHC Ib). MHC Ib molecules are structurally related to class Ia proteins because they show a typical (α1–α2) MHC fold on a single polypeptide, which, in the case of Ib, does not pair up obligatorily with β2-microglobulin (Rodgers & Cook, 2005). Furthermore, although many MHC Ib genes are also located in the MHC locus, they tend to be oligomorphic—few alleles exist in the population—which is in marked contrast with the extensive polymorphism of class Ia. The oligomorphism of class Ib might therefore be an advantage for the design of cancer therapies with a wider application within the cancer patient population. Amino-acid sequence identity/homology is not a useful criterion to establish relationships between class Ib members, as functional equivalents across species—for example, proteins that bind to a given receptor—are often not orthologous. Some class Ib genes are in fact more closely related to class Ia genes in evolutionary terms than to other class Ib members (Rodgers & Cook, 2005). MHC Ib proteins have been largely shown to bind to stimulatory or inhibitory receptors expressed on T, NK and/or NKT lymphocytes (Fig 1); there are, however, a few exceptions such as the haemochromatosis antigen (HFE) or the neonatal Fc receptor (FcRn).
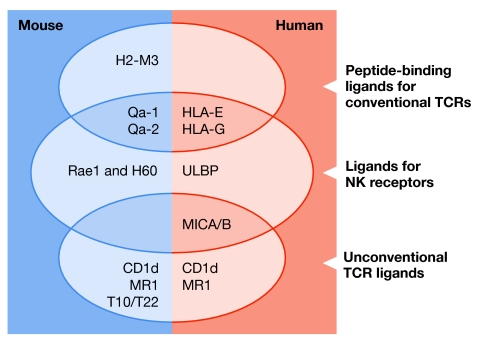
Figure 1.
Major histocompatibility complex class Ib proteins bind various immunoreceptors. Listed are major histocompatibility complex class Ib (MHC Ib) molecules for which receptor binding has been well characterized. Conventional T-cell receptors (TCRs) are those of polyclonal αβ T cells; unconventional TCRs correspond to oligoclonal T-cell subsets, such as natural killer (NK) T cells (for CD1d), γδ T cells (T10/T22, MICA/B) or gut-associated T cells (MR1). Proposed functional homologues between mice and humans are in the same row. H60, histocompatibility antigen 60; HLA, human leukocyte antigen; MICA/B, MHC class I chain-related peptides A/B; Rae1, retinoic acid early inducible gene 1; ULBP, UL16 binding protein.
Here, we focus on the ability of some MHC Ib proteins to act as ‘reporters' of cellular transformation and trigger anti-tumour immune responses. We have selected three examples to illustrate the various mechanisms by which MHC Ib can activate or inhibit anti-tumour lymphocytes.
Qa-2 and HLA-G present peptides to CD8+ T cells
Murine Qa-2 and human leukocyte antigen (HLA)-G are ‘young' MHC Ib proteins in that they diverged from MHC Ia less than 20 million years ago, and therefore have many similarities with the classical Ia molecules: they present a repertoire of nonameric peptides, contain a CD8 binding loop, associate with β2-microglobulin (β2m), display similar exon–intron organization and even share a high degree of amino-acid sequence homology (Seliger et al, 2003).
The murine Qa-2 region, located in the H2-Q class Ib locus, encodes the proteins Q8 and Q9 in C57BL/6 (B6) mice, which differ from each other by 20 amino acids located in the α1 and α2 domains. These proteins have overlapping peptide-binding motifs and are recognized by cross-reactive anti-tumour CTLs, and therefore appear to be functionally equivalent (Chiang & Stroynowski, 2006). Surface expression of Qa-2 molecules requires a functional TAP (transporter associated with antigen processing) peptide transporter (Tabaczewski et al, 1994) and is dependent on ERAP1 (endoplasmic-reticulum-associated peptidase 1) processing, suggesting that peptides loaded onto Qa-2 molecules are generated through the conventional class Ia antigen-processing pathway (Yan et al, 2006). Qa-2 transcripts are widely expressed at low levels in healthy tissues, both in haematopoietic and in non-haematopoietic cell types (Ungchusri et al, 2001). However, Q9 expression was found to be lost or severely reduced in a large panel of in vivo-derived tumour cell lines, including cells derived from T- and B-cell lymphoma, mastocytoma, melanoma and hepatoma (Ungchusri et al, 2001). Serological and reverse-transcriptase-PCR (RT-PCR) analyses have also shown that the primary B16 melanoma tumour cell line and all its variants had no Q8 and Q9 expression, whereas the control melanocyte line was positive for these antigens (Chiang et al, 2003). These results suggest that Q8 and Q9 expression is silenced early during tumour progression.
Several in vitro and in vivo studies have nonetheless shown Q9 involvement in tumour rejection. Syngeneic B6 mice injected with the B78H1 melanoma cell line—selectively devoid of TAP2 and class Ia (Kb and Db) transcripts and genetically manipulated to re-express Q9 on its surface—were protected from melanoma outgrowth, unlike mice challenged with class Ia-negative B78H1 or with TAP2-transfected parental melanoma cells (Chiang et al, 2003). Subsequent studies have shown CD8+ CTL involvement in Q9-mediated protection from melanoma development, as protection is lost in CD8 knockout and severe combined immunodeficiency (SCID) mice, but not in CD4 knockouts (Chiang & Stroynowski, 2004). Furthermore, it was demonstrated that mice surviving the original challenge became resistant to subsequent doses of Q9-positive melanoma, suggesting that Qa-2 acted as a restriction element for anti-tumour CD8+T cells, and that both effector and memory cells were generated in immunized mice (Chiang & Stroynowski, 2004). Q9-restricted CTL responses able to recognize lung carcinoma and T-cell lymphoma have also been observed (Chiang & Stroynowski, 2005). In addition, Q9 was shown to act as a restriction element for a tumour antigen common to these tumours and melanoma. It was observed that CTL clones raised in response to a challenge with Q9-expressing 3LLA9F1 lung carcinoma, RMA T-cell lymphoma or GMQ9TAP B78H1 melanoma efficiently killed all of these tumours in cytotoxicity assays in a Q9-restricted manner. This also suggests that CTLs generated in the primary response establish a pool of memory cells that exhibit cross-reactivity against various tumours (Chiang & Stroynowski, 2005). Qa-2 proteins are also recognized by NK lymphocytes, and these have been shown to be essential for the rejection of large doses of Q9-positive melanoma cells even in the presence of a CTL response (Chiang et al, 2003). Therefore, it will be important to analyse the interplay between CD8+ T and NK cells in Qa-2-dependent anti-tumour responses.
Although there is no human orthologue of Qa-2, some have suggested HLA-G is its functional homologue as both proteins have immunoregulatory roles, are involved in embryonic development, and exist in membrane-bound and soluble forms that arise by alternative splicing (Comiskey et al, 2003). HLA-G expression is augmented in various tumours, including melanomas, breast, renal, ovarian and lung carcinomas, gliomas, B and T non-Hodgkin lymphomas, acute leukaemias and colorectal cancers (Rouas-Freiss et al, 2005). However, it is important to note that HLA-G expression is not detected in all tumours; for example, HLA-G expression has been reported in ocular tumours such as retinoblastoma, whereas it has not been detected in uveal melanomas, even after treatment with interferon-γ (IFN-γ; Hurks et al, 2001). HLA-G expression is highly inducible and cytokines, such as interleukin (IL)-10, leukaemia inhibitory factor (LIF), tumour necrosis factor-α (TNF-α) and IFN-γ, differentially regulate its transcription in several tumour cell lines (Carosella & Dausset, 2003). Stress-inducing factors, such as heat shock and arsenite treatment, also induce HLA-G transcription in tumour cells (Ibrahim et al, 2000). Furthermore, it was recently shown that HLA-G expression is regulated by hypoxia in a hypoxia-inducible factor 1 (HIF1)-dependent manner (Mouillot et al, 2007). HLA-G1 protein expression can also be controlled at the post-translational level because its levels on the cell surface of carcinoma and melanoma cells are reduced upon activation of the NF-κB signalling pathway (Zidi et al, 2006). Unlike HLA class I genes, HLA-G expression is controlled by cis-acting epigenetic mechanisms, such as DNA methylation/demethylation and histone deacetylation/acetylation (Chang et al, 2003; Guillaudeux et al, 1995; Mouillot et al, 2005). For example, when cells of HLA-G positive melanoma tumours are subject to long-term in vitro culture, their HLA-G expression levels decrease over time, correlating with methylation of the HLA-G promoter. This suggests that lack of exposure to tumour microenvironmental conditions might lead to silencing of the gene (Chang et al, 2003).
Qa-2 expression in murine tumours makes them more susceptible to CTL-mediated lysis, whereas the aberrant expression of HLA-G antigens by human tumour cells seems to constitute an immune evasion mechanism. This would be analogous with their function in trophoblasts, which is to protect the fetus from maternal immune attack (Carosella et al, 2003). Indeed, several lines of evidence have shown that HLA-G can inhibit anti-tumour lymphocytes (Rouas-Freiss et al, 2005). First, HLA-G molecules were shown to interact directly with inhibitory receptors present on the surface of T (ILT-2) and NK cells (KIR2DL4, ILT-2), as well as monocytes/macrophages and dendritic cells (ILT-2 and ILT-4). Second, HLA-G molecules stabilize the cell surface expression of other non-classical HLA molecules such as HLA-E, which convey additional suppressive signals to lymphocytes through the widely expressed CD94/NKG2A killing inhibitory receptor. Third, HLA-G1 ligation decreases the secretion of IFN-γ and TNF-α, which are crucial for anti-tumour immunity (Rouas-Freiss et al, 2005). Fourth, antigen-presenting cells (APCs) that express HLA-G1 were shown to induce CD4+ T-cell anergy and to trigger their differentiation into suppressor cells that block CTL function (LeMaoult et al, 2004). This effect could either be a consequence of the interaction of HLA-G with an unknown receptor expressed by CD4+T cells, or an inhibitory effect of HLA-G on the APCs themselves. The HLA-G+ APCs present in cancer patients might thus be partly responsible for tumour immune escape owing to their suppressive properties. Furthermore, soluble HLA-G5 has been directly implicated in the inhibition of CD4+ and CD8+ T-cell proliferation. It causes cell cycle arrest by altering the balance between inhibitory molecules and cyclins, inducing the accumulation of p27kip and the reduction of cyclins D2, E, A and B (Bahri et al, 2006). Recently, it has also been shown that HLA-G molecules are transferred from tumour cells to activated NK cells by trogocytosis (Caumartin et al, 2007), which consists of the rapid transfer of intact cell-surface proteins between cells in contact with each other. The acquisition of HLA-G1 by NK cells blocks their proliferation and cytolytic function owing to its interaction with ILT2 on other NK cells and therefore induces a temporary state of immunosuppression in the NK-cell population (Caumartin et al, 2007). Consistent with its immuno-evasive role, HLA-G expression in some malignancies, such as colorectal cancers, correlates significantly with increased depth of invasion, histological grade, lymph nodal metastasis and clinical stages of the disease, suggesting that its presence could act as an independent prognostic factor for cancer patients (Ye et al, 2007).
Despite the vast amount of data, the role of HLA-G as an inhibitory ligand for anti-tumour lymphocytes remains controversial. For example, the main NK cell receptor for HLA-G, KIR2DL4, can also function as an activating receptor. Indeed, KIR2DL4 engagement has been reported either to inhibit cytotoxicity (Ponte et al, 1999) or to promote cytotoxicity and IFN-γ secretion (Kikuchi-Maki et al, 2003; Rajagopalan et al, 2001). Therefore, the elucidation of the functional consequences of HLA-G ligation, as well as the specific roles of membrane-bound and soluble forms of the protein (Morandi et al, 2007), require further investigation.
CD1d presents lipids to NKT cells
CD1d, a conserved member of the small CD1 protein family, presents lipid/glycolipid—instead of peptidic—antigens to TCR αβ complexes expressed by anti-tumour NKT cells in mice and humans (Godfrey et al, 2004). The dependence on CD1d for development and activation is the defining characteristic of NKT cells, which also express NK-cell-associated surface markers. NKT cell subsets can be further characterized by their TCR repertoire: type I NKT cells express an invariant TCR (using the Vα14Jα18 gene segments in mice and the Vα24 segment in humans), whereas type II cells display a more diverse repertoire (Godfrey et al, 2004). Although NKT cells are endowed with cytolytic factors that eliminate tumour cells such as perforin, Fas-ligand and TRAIL (tumour necrosis factor-related apoptosis inducing ligand) (Kawano et al, 1998), they also secrete large amounts of IFN-γ, which, in turn, stimulates anti-tumour NK and CD8+ CTLs (Smyth et al, 2002; Fig 2).
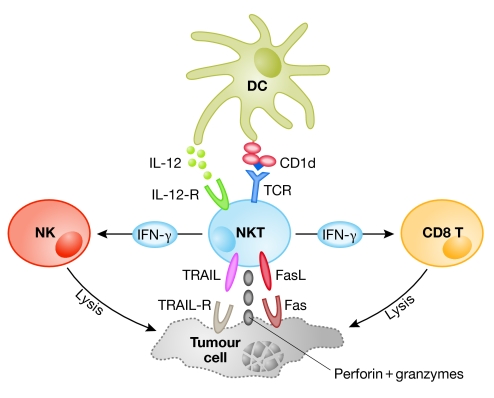
Figure 2.
Anti-tumour responses mediated by CD1d-activated type I natural killer T cells. After receiving activation signals from dendritic cells (DCs)—through CD1d and IL-12—NKT cells can either lyse tumour cells directly using the perforin/granzyme system or ligands for death receptors, or stimulate other cytotoxic cells such as NK and CD8+ T cells through IFN-γ secretion. IFN-γ, interferon-γ; IL-12, interleukin 12; NK, natural killer; NKT, natural killer T cell; TCR, T-cell receptor; TRAIL, tumour necrosis factor-related apoptosis inducing ligand.
CD1d can present various ligands to NKT cells (Zhou et al, 2004; Kinjo et al, 2005; Mattner et al, 2005), including tumour-derived lipids and glycolipids (Brutkiewicz, 2006). For example, the disialoganglioside GD3 is strongly upregulated in some tumours such as melanomas and can be cross-presented by CD1d-expressing murine APCs to NKT cells (Wu et al, 2003). Furthermore, some glycolipid fractions of tumour cell membranes are presented by CD1d and activate NKT cells (Gumperz et al, 2000). However, it has also been shown that glycolipid shedding by tumour cells can inhibit CD1d-mediated recognition of the tumour target (Sriram et al, 2002). Although the repertoire of transformation-induced glycolipids presented by CD1d remains to be characterized, α-galactosylceramide (α-GalCer), which is extracted from the marine sponge Agelas mauritianus, is the most potent stimulator of NKT cells known so far and has proven to be effective against a wide range of tumours. α-GalCer protects against the development of primary sarcomas induced chemically or owing to a lack of genetic tumour suppression (Hayakawa et al, 2003), as well as against metastasis of multiple tumour cell lines: B16 (melanoma), EL4 (thymoma), Colon-26 (colon adenocarcinoma), 3LL (lung carcinoma), RM-1 (prostate carcinoma) and DA3 (mammary carcinoma) (Swann et al, 2004). In the absence of CD1d—as in CD1d−/− mice—methylcholanthrene-induced sarcomas developed faster and at a higher frequency than in wild-type mice (Crowe et al, 2002). Similar results were obtained using Jα18−/− mice (Smyth et al, 2000; Crowe et al, 2002), which selectively lack type I NKT cells, suggesting that these invariant lymphocytes are the crucial subset responsible for tumour suppression. Not unexpectedly, the transfer of wild-type type I NKT cells into Jα18−/− mice resulted in protection from tumour development. Murine NKT lymphocytes have been recently characterized as strikingly heterogeneous in their response to tumours; type I NKT lymphocytes eliminate CD1d+ cells, whereas type II NKT actually suppress tumour immunity in several mouse tumour models (Terabe et al, 2005). It has been shown that downregulation of tumour immunity by NKT cells might be accomplished through an IL-13 and TGF-β-dependent mechanism that shuts down anti-tumour CD8+ T lymphocytes (Terabe et al, 2003). Another group has also reported differential anti-tumour effects by subdividing NKT cells according to their tissue origin—thymus or liver—and CD4 expression (Crowe et al, 2005). Future research will therefore attempt to manipulate the balance between distinct NKT cell subsets towards the promotion of anti-tumour immunity.
Several studies of cancer patients suffering from a wide range of tumours have shown decreased numbers of NKT cells in the peripheral blood, as compared with healthy volunteers (Swann et al, 2004). Furthermore, deficient NKT-cell production of IFN-γ has been associated with the progression from pre-malignant to malignant multiple myeloma, and the NKT dysfunction could be corrected in vitro by culturing the NKT cells with α-GalCer-pulsed dendritic cells (Dhodapkar et al, 2003). After the disappointing outcome of the first clinical trial with soluble α-GalCer in patients with solid tumours (Giaccone et al, 2002), the use of α-GalCer-loaded dendritic cells has produced promising pre-clinical data in mice (Fujii et al, 2002) and in phase I human clinical trials (Nieda et al, 2004; Ishikawa et al, 2005); phase II trials are currently under way.
NKG2D ligands directly activate anti-tumour lymphocytes
The immunoreceptor NKG2D provides important stimulatory signals to NK and T cells (Bauer et al, 1999). In humans, NKG2D is constitutively expressed on the cell surface of NK, CD8+ T and γδ T lymphocytes, but is absent from CD4+ T cells and monocytes (Raulet, 2003). Several ligands for both the human and mouse NKG2D have been identified and, surprisingly, there is low amino-acid sequence homology between them. Human NKG2D binds to MHC I chain-related (MIC) peptides A and B (MICA and MICB), and to UL16-binding proteins (ULBP, members 1–4). Mouse NKG2D binds to retinoic acid early inducible gene 1 (Rae1), histocompatibility antigen 60 (H60), and murine UL16-binding protein-like transcript 1 (MULT1). MICA/B, H60 and MULT1 are transmembrane proteins, whereas ULBP1–4 and Rae1 localize to the cell surface using glycosylphosphatidylinositol (GPI) linkages. None of the NKG2D ligands bind to additional—peptide or lipid—antigens but rather interact directly with the receptor. In addition, in contrast to the MHC Ib molecules described above, NKG2D ligands do not associate with β2-microglobulin (Raulet, 2003). For some authors, the unorthodoxy of NKG2D ligands prompts their formal designation as ‘MHC class I-related' instead of MHC Ib (Sullivan et al, 2006).
The murine ligands Rae1 and H60 are rare in healthy adult tissues, but their transcription is strongly induced in keratinocytes after their in vivo exposure to carcinogens (Girardi et al, 2001), and they are overexpressed in the cutaneous papillomas and carcinomas that subsequently develop, as well as in other tumours (Table 1). The expression of Rae1 or H60 by target cells was shown to enhance cytolysis and the production of IFN-γ by anti-tumour CTLs (Diefenbach et al, 2000) and γδ T lymphocytes (Girardi et al, 2001), leading to tumour rejection in vivo. Furthermore, transfection of rae1 or h60 or mult1 into NK-cell-resistant target cells made them susceptible to NK-cell-mediated killing and stimulated IFN-γ secretion by NK cells (Carayannopoulos et al, 2002; Cerwenka et al, 2000; Diefenbach et al, 2001). However, recent data suggest that IFN-γ can paradoxically downregulate H60 expression in tumours (Bui et al, 2006). Future experiments should try to reconcile these findings and elucidate the dynamics of H60 during tumour development in vivo.
Table 1.
mRNA/protein expression patterns for NKG2D ligands
| NKG2D ligand | Basal | Augmented* |
|---|---|---|
| MICA/B (human) | Protein in gastrointestinal epithelium, endothelial cells, fibroblasts; mRNA in keratinocytes Soluble protein in placenta | Protein in melanoma, myeloma, lymphoma, and colon, breast, lung, liver, ovary, kidney and cervical carcinomas Soluble protein in gastrointestinal and prostate cancers, lymphomas and neuroblastoma |
| ULBP (human) | ULBP1, 2, 3 mRNA in heart, brain, lung, liver, thymus, testis, lymph nodes, tonsils and bone marrow ULBP4 mRNA mainly in skin and small intestine | Both mRNA and surface protein in melanoma, various leukaemias and carcinomas (stomach, colon, cervical and ovarian carcinomas for ULBP2 and ULBP3); ULBP1 and ULBP2 in T-cell lymphomas |
| Rae1 (mouse) | mRNA in embryonic tissues mRNA absent (or very rare) in healthy adult tissues | mRNA and surface protein in lymphomas, lung, prostate and cutaneous carcinomas (variable) |
| H60 (BALB/c mouse) | mRNA in embryonic tissues, adult thymus and spleen, but generally absent in healthy adult tissues | Both mRNA and surface protein in lymphomas, prostate and cutaneous carcinomas (variable) |
| Mult1 (mouse) | mRNA in thymus, spleen, lymph nodes, liver, gut, heart and lung | mRNA in T-cell lymphomas and sarcomas |
*‘Augmented' refers to transcription induction or increased surface protein expression, relative to ‘basal' levels present in healthy tissues (Raulet, 2003, and references therein). H60, histocompatibility antigen 60; MICA/B, MHC class I chain-related peptide A/B; MULT1, murine UL16-binding protein-like transcript 1; Rae1, retinoic acid early inducible gene 1; ULBP, UL16-binding protein.
The human MICA and MICB proteins—which are 91% identical at the amino-acid level—show a restricted and low expression in healthy tissues, but are strongly induced by cellular stress (including heat shock) and transformation, and accumulate in various tumour cell lines, particularly those of epithelial origin (Table 1). Atypically for MHC Ib molecules, the MIC genes are highly polymorphic: there are 61 MICA and 30 MICB alleles (Raulet, 2003). Although MICA/B was suggested to bind not only to NKG2D, but also to the Vγ1+ TCR expressed by human intraepithelial γδ T lymphocytes (Wu et al, 2002), this claim is highly controversial. In fact, the identity of the majority of γδ TCR ligands remains an unresolved biological issue in which MHC Ib proteins are expected to have a crucial role (Steele et al, 2000; Sullivan et al, 2006; Thedrez et al, 2007). The membrane-bound form of MICA provides stimulatory signals to killer lymphocytes, whereas a soluble version (sMICA), shed from the cell surface by matrix metalloproteinases, systemically downregulates surface NKG2D and impairs tumour cytolysis mediated by T and NK cells, therefore constituting an important immune evasion mechanism for tumours (Groh et al, 2002; Salih et al, 2002).
Distantly related to the MIC proteins are the members of the ULBP family (23–26% amino-acid identity). In contrast to Rae1 or MICA, ULBPs are expressed at significant levels in a wide range of healthy tissues and cell lines of both epithelial and nonepithelial origin (Table 1; Cosman et al, 2001). Ectopic expression of ULBP1 or ULBP2 on murine EL4 or RMA tumour cells elicits potent anti-tumour responses in syngeneic B6 and SCID mice, recruiting NK, NKT and T cells to the tumour (Sutherland et al, 2006). Similarly, tumour cells that are insensitive to NK cells can be lysed effectively when transfected with ULBPs (Kubin et al, 2001).
Conclusion
In summary, NKG2D recognition of target tumour cells constitutes a potent anti-tumour mechanism, but its clinical application depends largely on the control and manipulation of the expression of NKG2D ligands. Therefore, the mechanisms controlling their specific regulatory signals need to be elucidated.
The immune system ‘sculpts' tumours by selecting those that, owing to their reduced immunogenicity, escape its recognition/destruction. This process of elimination versus escape creates a dynamic relationship that has been termed ‘cancer immunoediting' (Dunn et al, 2002). Non-classical MHC proteins can function as indicators of cellular transformation to anti-tumour lymphocytes, either directly or through the presentation of endogenous antigens that are (over-)expressed in tumours. Therefore, silencing MHC Ib expression might be a major immune evasion mechanism used by tumours, as described for Qa-2 during melanoma progression (Chiang et al, 2003). Another immune evasion strategy that involves MHC Ib proteins consists on the generation of soluble protein versions that block or internalize the corresponding receptors on lymphocytes, as mentioned for MICA (Groh et al, 2002; Salih et al, 2002). Similarly, the sustained surface expression of murine Rae1 has also been shown to have an inhibitory effect on NKG2D-expressing anti-tumour lymphocytes (Ogasawara et al, 2003; Oppenheim et al, 2005). Furthermore, CD1d-restricted type II NKT cells downregulate anti-tumour immunity (Terabe et al, 2005), whereas type I NKT cells promote tumour elimination. Such behaviour highlights the importance of determining the specific signals delivered by MHC Ib proteins to manipulate them towards protection. We believe that a better understanding of the underlying regulatory mechanisms of MHC Ib will make it possible to engineer vaccination approaches against various cancers that take advantage of the oligomorphism of most MHC Ib molecules.

Anita Q. Gomes

Daniel V. Correia

Bruno Silva-Santos
Acknowledgments
We apologize to the authors whose work could not be cited owing to space constraints. We thank Fundação para a Ciência e Tecnologia of the Portuguese Ministry of Science and Technology [PTDC/SAU-MII/71662/2006] for support. B.S.-S. is a European Molecular Biology Organization (EMBO) Installation Grantee and acknowledges the EMBO's Young Investigator Programme.
References
- Bahri R, Hirsch F, Josse A, Rouas-Freiss N, Bidere N, Vasquez A, Carosella ED, Charpentier B, Durrbach A (2006) Soluble HLA-G inhibits cell cycle progression in human alloreactive T lymphocytes. J Immunol 176: 1331–1339 [DOI] [PubMed] [Google Scholar]
- Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T (1999) Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285: 727–729 [DOI] [PubMed] [Google Scholar]
- Brutkiewicz RR (2006) CD1d ligands: the good, the bad, and the ugly. J Immunol 177: 769–775 [DOI] [PubMed] [Google Scholar]
- Bui JD, Carayannopoulos LN, Lanier LL, Yokoyama WM, Schreiber RD (2006) IFN-dependent down-regulation of the NKG2D ligand H60 on tumours. J Immunol 176: 905–913 [DOI] [PubMed] [Google Scholar]
- Burnet M (1957) Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br Med J 1: 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carayannopoulos LN, Naidenko OV, Fremont DH, Yokoyama WM (2002) Cutting edge: murine UL16-binding protein-like transcript 1: a newly described transcript encoding a high-affinity ligand for murine NKG2D. J Immunol 169: 4079–4083 [DOI] [PubMed] [Google Scholar]
- Carosella ED, Dausset J (2003) Progress of HLA-G in cancer. Semin Cancer Biol 13: 315–316 [DOI] [PubMed] [Google Scholar]
- Carosella ED, Moreau P, Le Maoult J, Le Discorde M, Dausset J, Rouas-Freiss N (2003) HLA-G molecules: from maternal–fetal tolerance to tissue acceptance. Adv Immunol 81: 199–252 [DOI] [PubMed] [Google Scholar]
- Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, LeMaoult J (2007) Trogocytosis-based generation of suppressive NK cells. EMBO J 26: 1423–1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL (2000) Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity 12: 721–727 [DOI] [PubMed] [Google Scholar]
- Chang CC, Murphy SP, Ferrone S (2003) Differential in vivo and in vitro HLA-G expression in melanoma cells: potential mechanisms. Hum Immunol 64: 1057–1063 [DOI] [PubMed] [Google Scholar]
- Chiang EY, Stroynowski I (2004) A nonclassical MHC class I molecule restricts CTL-mediated rejection of a syngeneic melanoma tumour. J Immunol 173: 4394–4401 [DOI] [PubMed] [Google Scholar]
- Chiang EY, Stroynowski I (2005) Protective immunity against disparate tumours is mediated by a nonpolymorphic MHC class I molecule. J Immunol 174: 5367–5374 [DOI] [PubMed] [Google Scholar]
- Chiang EY, Stroynowski I (2006) The role of structurally conserved class I MHC in tumour rejection: contribution of the Q8 locus. J Immunol 177: 2123–2130 [DOI] [PubMed] [Google Scholar]
- Chiang EY, Henson M, Stroynowski I (2003) Correction of defects responsible for impaired Qa-2 class Ib MHC expression on melanoma cells protects mice from tumour growth. J Immunol 170: 4515–4523 [DOI] [PubMed] [Google Scholar]
- Comiskey M, Goldstein CY, De Fazio SR, Mammolenti M, Newmark JA, Warner CM (2003) Evidence that HLA-G is the functional homolog of mouse Qa-2, the Ped gene product. Hum Immunol 64: 999–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D, Mullberg J, Sutherland CL, Chin W, Armitage R, Fanslow W, Kubin M, Chalupny NJ (2001) ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity 14: 123–133 [DOI] [PubMed] [Google Scholar]
- Crowe NY, Smyth MJ, Godfrey DI (2002) A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med 196: 119–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ (2005) Differential antitumour immunity mediated by NKT cell subsets in vivo. J Exp Med 202: 1279–1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhodapkar MV, Krasovsky J, Osman K, Geller MD (2003) Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med 198: 1753–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH (2000) Ligands for the murine NKG2D receptor: expression by tumour cells and activation of NK cells and macrophages. Nat Immunol 1: 119–126 [DOI] [PubMed] [Google Scholar]
- Diefenbach A, Jensen ER, Jamieson AM, Raulet DH (2001) Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 413: 165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD (2002) Cancer immunoediting: from immunosurveillance to tumour escape. Nat Immunol 3: 991–998 [DOI] [PubMed] [Google Scholar]
- Fujii S, Shimizu K, Kronenberg M, Steinman RM (2002) Prolonged IFN-γ-producing NKT response induced with α-galactosylceramide-loaded DCs. Nat Immunol 3: 867–874 [DOI] [PubMed] [Google Scholar]
- Giaccone G et al. (2002) A phase I study of the natural killer T-cell ligand α-galactosylceramide (KRN7000) in patients with solid tumours. Clin Cancer Res 8: 3702–3709 [PubMed] [Google Scholar]
- Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC (2001) Regulation of cutaneous malignancy by γδ T cells. Science 294: 605–609 [DOI] [PubMed] [Google Scholar]
- Girardi M, Glusac E, Filler RB, Roberts SJ, Propperova I, Lewis J, Tigelaar RE, Hayday AC (2003) The distinct contributions of murine T cell receptor (TCR)γδ+ and TCRαβ+ T cells to different stages of chemically induced skin cancer. J Exp Med 198: 747–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L (2004) NKT cells: what's in a name? Nat Rev Immunol 4: 231–237 [DOI] [PubMed] [Google Scholar]
- Groh V, Wu J, Yee C, Spies T (2002) Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419: 734–738 [DOI] [PubMed] [Google Scholar]
- Guillaudeux T, Rodriguez AM, Girr M, Mallet V, Ellis SA, Sargent IL, Fauchet R, Alsat E, Le Bouteiller P (1995) Methylation status and transcriptional expression of the MHC class I loci in human trophoblast cells from term placenta. J Immunol 154: 3283–3299 [PubMed] [Google Scholar]
- Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, Behar SM (2000) Murine CD1d-restricted T cell recognition of cellular lipids. Immunity 12: 211–221 [DOI] [PubMed] [Google Scholar]
- Hayakawa Y, Rovero S, Forni G, Smyth MJ (2003) α-Galactosylceramide (KRN7000) suppression of chemical- and oncogene-dependent carcinogenesis. Proc Natl Acad Sci USA 100: 9464–9469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurks HM, Valter MM, Wilson L, Hilgert I, van den Elsen PJ, Jager MJ (2001) Uveal melanoma: no expression of HLA-G. Invest Ophthalmol Vis Sci 42: 3081–3084 [PubMed] [Google Scholar]
- Ibrahim EC, Morange M, Dausset J, Carosella ED, Paul P (2000) Heat shock and arsenite induce expression of the nonclassical class I histocompatibility HLA-G gene in tumour cell lines. Cell Stress Chaperones 5: 207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, Iizasa T, Nakayama T, Taniguchi M, Fujisawa T (2005) A phase I study of α-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res 11: 1910–1917 [DOI] [PubMed] [Google Scholar]
- Kawano T et al. (1998) Natural killer-like nonspecific tumour cell lysis mediated by specific ligand-activated Vα14] NKT cells. Proc Natl Acad Sci USA 95: 5690–5693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi-Maki A, Yusa S, Catina TL, Campbell KS (2003) KIR2DL4 is an IL-2-regulated NK cell receptor that exhibits limited expression in humans but triggers strong IFN-γ production. J Immunol 171: 3415–3425 [DOI] [PubMed] [Google Scholar]
- Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M (2005) Recognition of bacterial glycosphingolipids by natural killer T cells. Nature 434: 520–525 [DOI] [PubMed] [Google Scholar]
- Kubin M, Cassiano L, Chalupny J, Chin W, Cosman D, Fanslow W, Mullberg J, Rousseau AM, Ulrich D, Armitage R (2001) ULBP1, 2, 3: novel MHC class I-related molecules that bind to human cytomegalovirus glycoprotein UL16, activate NK cells. Eur J Immunol 31: 1428–1437 [DOI] [PubMed] [Google Scholar]
- LeMaoult J, Krawice-Radanne I, Dausset J, Carosella ED (2004) HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci USA 101: 7064–7069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattner J et al. (2005) Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434: 525–529 [DOI] [PubMed] [Google Scholar]
- Morandi F, Levreri I, Bocca P, Galleni B, Raffaghello L, Ferrone S, Prigione I, Pistoia V (2007) Human neuroblastoma cells trigger an immunosuppressive program in monocytes by stimulating soluble HLA-G release. Cancer Res 67: 6433–6441 [DOI] [PubMed] [Google Scholar]
- Mouillot G, Marcou C, Rousseau P, Rouas-Freiss N, Carosella ED, Moreau P (2005) HLA-G gene activation in tumour cells involves cis-acting epigenetic changes. Int J Cancer 113: 928–936 [DOI] [PubMed] [Google Scholar]
- Mouillot G, Marcou C, Zidi I, Guillard C, Sangrouber D, Carosella ED, Moreau P (2007) Hypoxia modulates HLA-G gene expression in tumour cells. Hum Immunol 68: 277–285 [DOI] [PubMed] [Google Scholar]
- Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ (2004) Therapeutic activation of Vα24+Vβ11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood 103: 383–389 [DOI] [PubMed] [Google Scholar]
- Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, Carnaud C, Bluestone JA, Lanier LL (2003) Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity 18: 41–51 [DOI] [PubMed] [Google Scholar]
- Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC (2005) Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumour immunosurveillance. Nat Immunol 6: 928–937 [DOI] [PubMed] [Google Scholar]
- Ponte M, Cantoni C, Biassoni R, Tradori-Cappai A, Bentivoglio G, Vitale C, Bertone S, Moretta A, Moretta L, Mingari MC (1999) Inhibitory receptors sensing HLA-G1 molecules in pregnancy: decidua-associated natural killer cells express LIR-1 and CD94/NKG2A and acquire p49, an HLA-G1-specific receptor. Proc Natl Acad Sci USA 96: 5674–5679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Fu J, Long EO (2001) Cutting edge: induction of IFN-γ production but not cytotoxicity by the killer cell Ig-like receptor KIR2DL4 (CD158d) in resting NK cells. J Immunol 167: 1877–1881 [DOI] [PubMed] [Google Scholar]
- Raulet DH (2003) Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol 3: 781–790 [DOI] [PubMed] [Google Scholar]
- Rodgers JR, Cook RG (2005) MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol 5: 459–471 [DOI] [PubMed] [Google Scholar]
- Rouas-Freiss N, Moreau P, Ferrone S, Carosella ED (2005) HLA-G proteins in cancer: do they provide tumour cells with an escape mechanism? Cancer Res 65: 10139–10144 [DOI] [PubMed] [Google Scholar]
- Salih HR, Rammensee HG, Steinle A (2002) Cutting edge: down-regulation of MICA on human tumours by proteolytic shedding. J Immunol 169: 4098–4102 [DOI] [PubMed] [Google Scholar]
- Seliger B, Abken H, Ferrone S (2003) HLA-G and MIC expression in tumours and their role in anti-tumour immunity. Trends Immunol 24: 82–87 [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI (2000) Differential tumour surveillance by natural killer (NK) and NKT cells. J Exp Med 191: 661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Crowe NY, Godfrey DI (2001) NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol 13: 459–463 [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI (2002) Sequential production of interferon-γ by NK1.1+ T cells and natural killer cells is essential for the antimetastatic effect of α-galactosylceramide. Blood 99: 1259–1266 [DOI] [PubMed] [Google Scholar]
- Sriram V, Cho S, Li P, O'Donnell PW, Dunn C, Hayakawa K, Blum JS, Brutkiewicz RR (2002) Inhibition of glycolipid shedding rescues recognition of a CD1+ T cell lymphoma by natural killer T (NKT) cells. Proc Natl Acad Sci USA 99: 8197–8202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CR, Oppenheim DE, Hayday AC (2000) γδ T cells: non-classical ligands for non-classical cells. Curr Biol 10: R282–R285 [DOI] [PubMed] [Google Scholar]
- Street SE, Hayakawa Y, Zhan Y, Lew AM, MacGregor D, Jamieson AM, Diefenbach A, Yagita H, Godfrey DI, Smyth MJ (2004) Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and γδ T cells. J Exp Med 199: 879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan LC, Hoare HL, McCluskey J, Rossjohn J, Brooks AG (2006) A structural perspective on MHC class Ib molecules in adaptive immunity. Trends Immunol 27: 413–420 [DOI] [PubMed] [Google Scholar]
- Sutherland CL, Rabinovich B, Chalupny NJ, Brawand P, Miller R, Cosman D (2006) ULBPs, human ligands of the NKG2D receptor, stimulate tumour immunity with enhancement by IL-15. Blood 108: 1313–1319 [DOI] [PubMed] [Google Scholar]
- Swann J, Crowe NY, Hayakawa Y, Godfrey DI, Smyth MJ (2004) Regulation of antitumour immunity by CD1d-restricted NKT cells. Immunol Cell Biol 82: 323–331 [DOI] [PubMed] [Google Scholar]
- Tabaczewski P, Shirwan H, Lewis K, Stroynowski I (1994) Alternative splicing of class Ib major histocompatibility complex transcripts in vivo leads to the expression of soluble Qa-2 molecules in murine blood. Proc Natl Acad Sci USA 91: 1883–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabe M et al. (2003) Transforming growth factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumour immunosurveillance: abrogation prevents tumour recurrence. J Exp Med 198: 1741–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, Godfrey DI, Ostrand-Rosenberg S, Smyth MJ, Berzofsky JA (2005) A nonclassical non-Vα14Jα18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumour immunosurveillance. J Exp Med 202: 1627–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thedrez A, Sabourin C, Gertner J, Devilder MC, Allain-Maillet S, Fournie JJ, Scotet E, Bonneville M (2007) Self/non-self discrimination by human γδ T cells: simple solutions for a complex issue? Immunol Rev 215: 123–135 [DOI] [PubMed] [Google Scholar]
- Ungchusri T, Chiang EY, Brown G, Chen M, Tabaczewski P, Timares L, Stroynowski I (2001) Widespread expression of the nonclassical class I Qa-2 antigens in hemopoietic and nonhemopoietic cells. Immunogenetics 53: 455–467 [DOI] [PubMed] [Google Scholar]
- Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB (2003) Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med 198: 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Groh V, Spies T (2002) T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial γδ T cells. J Immunol 169: 1236–1240 [DOI] [PubMed] [Google Scholar]
- Yan J, Parekh VV, Mendez-Fernandez Y, Olivares-Villagomez D, Dragovic S, Hill T, Roopenian DC, Joyce S, Van Kaer L (2006) In vivo role of ER-associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J Exp Med 203: 647–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SR, Yang H, Li K, Dong DD, Lin XM, Yie SM (2007) Human leukocyte antigen G expression: as a significant prognostic indicator for patients with colorectal cancer. Mod Pathol 20: 375–383 [DOI] [PubMed] [Google Scholar]
- Zhou D et al. (2004) Lysosomal glycosphingolipid recognition by NKT cells. Science 306: 1786–1789 [DOI] [PubMed] [Google Scholar]
- Zidi I, Guillard C, Marcou C, Krawice-Radanne I, Sangrouber D, Rouas-Freiss N, Carosella ED, Moreau P (2006) Increase in HLA-G1 proteolytic shedding by tumour cells: a regulatory pathway controlled by NF-κB inducers. Cell Mol Life Sci 63: 2669–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]