Abstract
IQGAPs are actin-binding proteins that scaffold numerous interaction partners, transmitting extracellular signals that influence mitogenic, morphological and migratory cell behaviour. However, the precise mechanisms by which IQGAP proteins influence actin dynamics and actin filament structures have been elusive. Now that IQGAP1 has emerged as a potential key regulator of actin-cytoskeletal dynamics by recruiting both the actin related protein (Arp)2/3 complex and/or formin-dependent actin polymerizing machineries, we propose that IQGAP1 might coordinate the function of mechanistically different actin nucleators for cooperative localized actin filament production in various cellular processes.
Keywords: IQGAP, formins, N-WASp, Arp2/3, actin
Introduction
IQGAP1 was initially identified as a Rac and Cdc42 binding protein that associates with actin and localizes to actin-rich structures such as lamellipodia, membrane ruffles and cell–cell contacts (Hart et al, 1996; Kuroda et al, 1996). The term IQGAP is derived from the protein structure, which includes calmodulin-binding IQ motifs and that has homology to GTPase activating proteins (GAPs) (Weissbach et al, 1994). IQGAPs are evolutionarily conserved multidomain proteins containing several protein interaction motifs that can mediate binding to a diverse set of target proteins. Three isoforms have been described so far in mammals (Brown & Sacks, 2006; Wang et al, 2007) and, although they share a high degree of sequence homology, they differ in tissue distribution and function. Protein expression analysis indicates that IQGAP1 is present in all tissues tested, whereas the other two isoforms show a more distinct tissue distribution: IQGAP3 is enriched in brain and lung tissue, whereas IQGAP2 is more abundant in the liver and stomach (Wang et al, 2007), and can be detected in platelets (Schmidt et al, 2003).
It is well established that IQGAPs inhibit the GTPase activity of Cdc42 and Rac1 to stabilize their GTP-bound form (Brill et al, 1996; Hart et al, 1996; Noritake et al, 2004), which is consistent with their involvement in actin-dependent functions such as cell shape and motility (Table 1). IQGAP proteins have an important role in cytokinesis in yeast and Dictyostelium (Machesky, 1998). In addition, IQGAPs are involved in the formation of cell–cell adhesion sites through binding to E-cadherins as well as β-catenins (Noritake et al, 2005), and regulate cell adhesion through binding to Rap1 (Jeong et al, 2007). IQGAP proteins also participate in cancer cell invasion and metastasis, their expression is upregulated in various invasive human cancers and elevated levels of IQGAP1 have been associated with a poor prognosis (McDonald et al, 2007; Dong et al, 2006). Another principal role of IQGAP seems to be its function as a scaffold for the mitogen-activated protein (MAP) kinase pathway, exerted through interactions with B-Raf, extracellular signal-regulated kinase 2 (Erk2) as well as MAPK/ERK kinase (MEK1/2) (Ren et al, 2007; Roy et al, 2005).
Table 1.
Cellular localizations implicated in the cytoskeletal functions of IQGAP proteins
| Organism and IQGAP proteins | Cellular localizations | Proposed cytoskeletal functions | References |
|---|---|---|---|
| Saccharomyces cerevisiae | |||
| Iqg1/Cyk1 | Bud neck Actomyosin ring | Cytokinesis and cell polarity Cytokinesis | Osman & Cerione, 1998 Shannon & Li, 1999 |
| Schizosaccharomyces pombe | |||
| Rng2 | Actomyosin ring | Cytokinesis | Eng et al, 1998 |
| Dictyostelium discoideum | |||
| DGAP1 | Cell cortex, cleavage furrow | Cytokinesis | Faix et al, 2001 |
| Xenopus laevis | |||
| XIQGAP1 | Filopodia, lamellipodia, cell–cell contacts | Cell migration | Yamashiro et al, 2003 |
| XIQGAP2 | Nucleus, adherens junctions | E-cadherin-mediated cell–cell contacts | Yamashiro et al, 2003, 2007 |
| Mammalian | |||
| IQGAP1 | Lamellipodia Adherens junctions Leading front Pseudopodia Phagocytic cup | Actin crosslinking/bundling E-cadherin-mediated cell–cell contacts Microtubule capture, polarity Cell motility, invasion Phagocytosis | Bashour et al, 1997 Kuroda et al, 1998 Fukata et al, 2002 Jia et al, 2005 Brandt et al, 2007 |
| IQGAP2 | Filopodia, lamellipodia | Platelet aggregation | Schmidt et al, 2003 |
| IQGAP3 | Tip of axons | Axon elongation | Wang et al, 2007 |
IQGAPs, proteins containing calmodulin-binding IQ motifs and with homology to GTPase activating proteins (GAPs).
Regulation of IQGAP activity
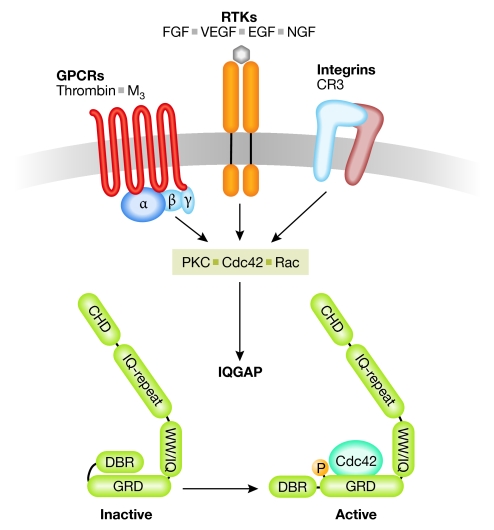
It is widely accepted that the subcellular localization of IQGAPs is controlled by Cdc42 and/or Rac activity (Watanabe et al, 2004). However, another important mechanism that regulates the function of IQGAPs is phosphorylation. IQGAP1 has recently been shown to be a protein kinase C (PKC) ɛ substrate in vivo and in vitro, and PKC-dependent phosphorylation regulates the conformation of the IQGAP1 carboxyl terminus to facilitate Cdc42 binding. Interestingly, it has been proposed that IQGAP1 is regulated by autoinhibition and that phosphorylation of Ser 1443 relieves the autoinhibited fold (Fig 1; Grohmanova et al, 2004). In agreement with this finding, a phosphomimetic variant of IQGAP1 stimulates neurite outgrowth in NIE-115 cells (Li et al, 2005), indicating that phosphorylation controls IQGAP1 function towards the cytoskeleton. However, it is not clear whether phosphorylation-dependent regulation is restricted to IQGAP1 as its role for other isoforms has not yet been investigated.
Figure 1.
Upstream regulators of IQGAP proteins. Various plasma membrane receptors such as G-protein-coupled receptors (GPCRs), receptor tyrosine kinases (RTKs) and integrins signal towards IQGAP (green) proteins through direct interaction or the activation of small GTPases and protein kinase C (PKC). IQGAP becomes activated through phosphorylation and conformational changes at its carboxyl terminus. CHD, calponin homology domain; CR3, complement receptor 3; DBR, diaphanous binding region; EGF, epidermal growth factor; FGF, fibroblast growth factor; GRD, Gap related domain; IQGAPs, proteins containing calmodulin-binding IQ motifs and with homology to GTPase activating proteins (GAPs); NGF, nerve growth factor; VEGF, vascular endothelial growth factor; WW, domain with two conserved Trp (W) residues.
IQGAPs have been implicated in signalling downstream from receptor tyrosine kinases such as the epidermal growth factor (EGF), nerve growth factor (NGF), fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) receptors, as well as G-protein-coupled receptors such as the thrombin or the M3 receptor (Bensenor et al, 2007; Brown & Sacks, 2006; Wang et al, 2007). In addition, IQGAP1 recruitment to complement receptor 3 (CR3) receptors during phagocytosis has also been reported (Brandt et al, 2007), indicating that some integrins might act upstream from IQGAP proteins. Differential receptor signalling might therefore regulate the subcellular localization of IQGAPs in response to various extracellular cues (Table 1; Fig 1). This possibility raises the question of whether IQGAPs scaffold different sets of ‘effector' proteins or whether binding to the same set of proteins occurs within a particular location in the cell.
IQGAP proteins and microtubule dynamics
IQGAP1 regulates microtubule tip proteins during polarized cell migration. At the leading edge of migrating cells, IQGAP1 binds to the cytoplasmic linker protein 170 (CLIP170) and bridges microtubule plus ends to the actin meshwork for cortical polarity (Fukata et al, 2002). In simple wound healing assays, IQGAP1 links Cdc42–Rac1 signalling and actin filaments during polarization and migration of fibroblasts through its C-terminal interaction with adenomatous polyposis coli (APC) (Watanabe et al, 2004); therefore, IQGAP proteins are critical mediators of actin microtubule crosstalk, which might involve the defined spatiotemporal recruitment of other actin regulators. For example, in migrating cells, IQGAP1 is necessary for the localization of the actin nucleator Diaphanous 1 (Dia1) to the leading edge (Brandt et al, 2007), which has also been implicated in stabilizing microtubules at the cell front through microtubule end-binding protein 1 (EB1) and APC interactions (Wen et al, 2004). This suggests a scenario in which IQGAP1-mediated localization of Dia1 activity could be required for the selective stabilization of captured microtubules. The potential role of localized actin assembly for actin–microtubule interactions at the cell cortex requires further analysis. One might speculate that the role of IQGAPs in microtubule capture—through interactions with CLIP170 and APC—could be involved in the spatiotemporal control of formin functions.
IQGAPs as actin-binding and crosslinking proteins
Despite the evidence that has accumulated in recent years about the role of IQGAPs in cortical microtubule capture, they now re-emerge as important integrators for actin dynamics. Initial observations for the role of IQGAPs as actin cytoskeletal regulators arose from studies by Bloom and colleagues, who co-purified IQGAP1 from cytosolic F-actin fractions as a protein with actin crosslinking activity (Bashour et al, 1997). It was shown that this activity involves IQGAP1 oligomerization (Fukata et al, 1997) and later that a single, monomeric calponin homology domain (CHD) of IQGAP1 binds to actin with high affinity (Mateer et al, 2004). Consistently, IQGAP3 also interacts with actin filaments in the brain through its CHD (Wang et al, 2007); therefore, interactions of IQGAP with F-actin seem to be involved in actin crosslinking and/or bundling (Bensenor et al, 2007).
Interestingly, in budding yeasts IQGAPs are essential for actin accumulation at specific sites of high actin turnover—such as the cytokinetic ring—and the IQGAP homologue Cyk1/Iqg1 (cytokinesis protein 1/IQGAP-related protein1) is sequentially recruited before actin filaments become visible (Lippincott & Li, 1998). This also suggests that F-actin binding of IQGAP proteins is not involved in their subcellular localization but that it might be important for subsequent localized actin assembly. Indeed, Cyk1/Iqg1 has an important function for the assembly and contraction of the actomyosin ring, probably through the recruitment of actin to these structures. A mutant variant of Iqg1 that lacks the C terminus is unable to promote cytokinesis, although actin binding and localization to the actomyosin ring is unaffected, which indicates that the recruitment of additional factors is crucial for IQGAP function in yeast (Shannon & Li, 1999). Consistent with this, depletion of IQGAP proteins in budding yeast, Xenopus laevis and mammalian epithelial cells results in a loss of locally organized actin filaments at the cytokinetic ring or at cell adhesion sites, respectively (Lippincott & Li, 1998; Osman & Cerione, 1998; Yamashiro et al, 2007; Noritake et al, 2004), suggesting that IQGAPs are important for spatiotemporal F-actin assembly. Actin filament binding to IQGAP proteins is further controlled by intracellular calcium, which supports the dynamic regulation of IQGAP association with the cytoskeleton (Mateer et al, 2002; Kholmanskikh et al, 2006).
Is IQGAP a master regulator for localized F-actin assembly?
Numerous studies have suggested a role for IQGAP proteins in actin dynamics. In fact, one of the first biological functions described for IQGAP was its involvement in cytokinesis in various organisms (Machesky, 1998)—a conserved function that is strikingly shared with formins (Faix & Grosse, 2006). As IQGAP accumulates at the beginning of contractile ring formation in fission yeast (Wu & Pollard, 2005; Wu et al, 2006), it would be interesting to determine whether IQGAP proteins are involved in formin regulation during cytokinesis.
The specific role of IQGAP in local actin polymerization is not well understood. Some evidence was obtained from studies in sea urchin eggs in which actin polymerization from GTP-loaded Cdc42 affinity beads was found to be dependent on the presence of an IQGAP-related protein, which localized to the cleavage furrow of dividing eggs (Nishimura & Mabuchi, 2003). Izumi et al (2004) used adherens junction membrane preparations to show that F-actin levels increased when recombinant IQGAP1 was added, but not with a mutant lacking the actin-binding CHD.
But how could IQGAP1 stimulate actin assembly? Two scenarios have now emerged that provide a mechanistic insight into this question. IQGAP1 can steer and promote actin nucleation through interactions with two different and potent actin-assembling machineries: the actin related protein (Arp)2/3 complex and formins. This is particularly intriguing because these two actin-polymerizing mechanisms have been thought to fulfil independent functions for entirely different actin filament structures. Formins nucleate actin through their formin homology (FH) 2 domain to produce linear filaments through processive barbed end elongation, whereas Arp2/3 generates branched actin meshworks from pre-exisiting filaments with the help of nucleation-promoting factors such as the Wiskott–Aldrich syndrome protein (WASp; Pollard, 2007).
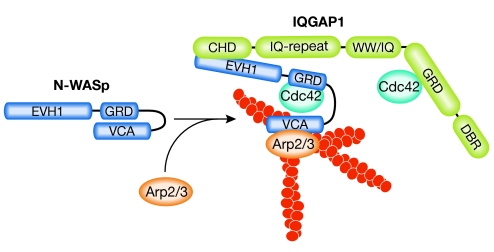
Through direct interaction and activation of neural-WASp (N-WASp), IQGAP1 can stimulate Arp2/3-dependent actin polymerization to generate branched actin filaments in vitro (Le Clainche et al, 2007; Bensenor et al, 2007), although the IQGAP domains responsible for N-WASp binding are still being debated. As IQGAP1 also stabilizes GTP-bound Cdc42—which is known to stimulate N-WASp—one could imagine that Arp2/3-dependent actin assembly is more efficient in this scenario (Fig 2).
Figure 2.
Model of IQGAP1-dependent regulation of N-WASp and Arp2/3. N-WASp (dark blue) is shown in its autoinhibited conformation. IQGAP1 (green) and GTP-Cdc42 bind to N-WASp to relieve autoinhibition. The open conformation of N-WASp stimulates the Arp2/3 complex and promotes branched actin filament polymerization. In addition, IQGAP1 might also support the open conformation of N-WASp through stabilization of active Cdc42. Arp2/3, actin related protein 2/3; CHD, calponin homology domain; DBR, diaphanous binding region; EVH1, Ena/VASP-homology 1; GRD, Gap related domain; IQGAPs, proteins containing calmodulin-binding IQ motifs and with homology to GTPase activating proteins (GAPs); N-WASp, neural-Wiskott–Aldrich syndrome protein; VCA, verprolin-connecting-acidic domain; WW, domain with two conserved Trp (W) residues.
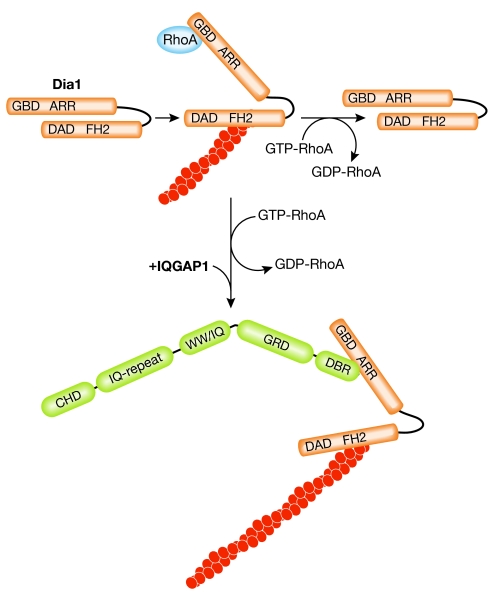
In a second mechanism, IQGAP1 was identified as a high-affinity binding partner for Dia1. Dia1 belongs to the group of diaphanous formins that, on Rho GTPase-induced release of autoinhibition, typically assemble unbranched actin filaments (Faix & Grosse, 2006). IQGAP1 interacts specifically with the RhoA-activated form of Dia1 and recruits it to local sites of actin assembly such as the leading front of migrating cells or the phagocytic cup (Brandt et al, 2007). Although the Dia1-binding region (DBR) of IQGAP1 does not activate the actin nucleation activity of Dia1 in vitro, it might function to stabilize its active conformation by preventing the autoinhibitory interaction between the diaphanous inhibitory domain (DID) and the diaphanous autoregulatory domain (DAD; Fig 3). However, such a scenario raises the question of how this multiprotein complex behaves during filament elongation, because diaphanous formins remain attached to the barbed ends during actin polymerization (Pruyne et al, 2002). Single-molecule imaging of IQGAP could clarify this. Nevertheless, the fact that IQGAPs recruit formin activity might help to explain their role in other actin-dependent processes such as cytokinesis.
Figure 3.
Model of IQGAP1-dependent regulation of Diaphanous 1. GTP-bound RhoA relieves autoinhibition of Dia1 (orange) between the diaphanous autoregulatory domain (DAD) and the armadillo repeat region (ARR). RhoA-GTP is rapidly hydrolysed. In the presence of IQGAP1 (green), the ARR of Dia1 binds to the diaphanous binding region (DBR) of IQGAP1 and stabilizes Dia1 in its active conformation. Dimerization of Dia1 and of IQGAP1 is not illustrated. CHD, calponin homology domain; FH2, formin homology 2; Dia1, Diaphanous 1; GBD, GTPase-binding domain; GRD, Gap related domain; IQGAPs, proteins containing calmodulin-binding IQ motifs and with homology to GTPase activating proteins (GAPs); WW, domain with two conserved Trp (W) residues.
Why does IQGAP1 directly control two potent actin nucleators that generate two different structures of actin filament networks? Strikingly, both Arp2/3 and Dia1 are involved in several actin-dependent processes such as dynamic E-cadherin-mediated cell–cell contacts (Sahai & Marshall, 2002; Verma et al, 2004; Drees et al, 2005), particle engulfment during phagocytosis (May et al, 2000; Colucci-Guyon et al, 2005) and T-cell migration (Zhang et al, 1999; Eisenmann et al, 2007; Sakata et al, 2007), which suggests that they function cooperatively and/or interdependently. One might therefore speculate that IQGAP1 acts as a platform at which Arp2/3 and Dia1-dependent actin polymerization converges. This possibility raises two crucial questions. Are these two actin nucleators recruited sequentially or simultaneously during dynamic processes in which actin nucleation is activated? Is the binding of the N-WASp–Arp2/3 complex and Dia1 to IQGAPs mutually exclusive or cooperative? Whether N-WASp interacts with the N or C terminus of IQGAP1 is controversial and the latter might preclude simultaneous binding to Dia1, at least in a monomeric IQGAP situation. Another interesting hypothesis is that F-actin binding of IQGAP1, or IQGAP1 stimulation of Dia1-mediated actin assembly, might facilitate the recruitment of N-WASp–Arp2/3 complexes to the proximity of pre-existing actin filaments, which are required for the initiation of Arp2/3-dependent actin nucleation (Pollard, 2007).
Concluding remarks
IQGAPs are emerging as crucial regulators of spatially defined actin assembly through the control of Dia1 and N-WASp activity. The accumulating evidence supporting the idea that formin and Arp2/3 cooperatively mediate active actin assembly in certain biological dynamic processes could help us to understand the role of both nucleators in cell shape and motility. Several cellular events involve the local control of actin polymerization, which, for specialized actin filament structures, might be coordinated through the cytoskeletal scaffolding IQGAPs by the integration and regulation of different protein complexes.

Dominique T. Brandt

Robert Grosse
Acknowledgments
We thank S. Marion, O.T. Fackler and laboratory members for critical comments and helpful discussions on the manuscript; we also thank R Jordan. This work was supported by the Emmy Noether Programme of the Deutsche Forschungsgemeinschaft (DFG) (GR 2111/1-2) to R.G.
References
- Bashour AM, Fullerton AT, Hart MJ, Bloom GS (1997) IQGAP1, a Rac- and Cdc42-binding protein, directly binds and cross-links microfilaments. J Cell Biol 137: 1555–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensenor LB, Kan HM, Wang N, Wallrabe H, Davidson LA, Cai Y, Schafer DA, Bloom GS (2007) IQGAP1 regulates cell motility by linking growth factor signaling to actin assembly. J Cell Sci 120: 658–669 [DOI] [PubMed] [Google Scholar]
- Brandt DT, Marion S, Griffiths G, Watanabe T, Kaibuchi K, Grosse R (2007) Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J Cell Biol 178: 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill S, Li S, Lyman CW, Church DM, Wasmuth JJ, Weissbach L, Bernards A, Snijders AJ (1996) The Ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin binding domain and interacts with calmodulin and Rho family GTPases. Mol Cell Biol 9: 4869–4878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MD, Sacks DB (2006) IQGAP1 in cellular signaling: bridging the GAP. Trends Cell Biol 16: 242–249 [DOI] [PubMed] [Google Scholar]
- Colucci-Guyon E, Niedergang F, Wallar BJ, Peng J, Alberts AS, Chavrier P (2005) A role for mammalian diaphanous-related formins in complement receptor (CR3)-mediated phagocytosis in macrophages. Curr Biol 15: 2007–2012 [DOI] [PubMed] [Google Scholar]
- Dong P, Nabeshima K, Nishimura N, Kawakami T, Hachisuga T, Kawarabayashi T, Iwasaki H (2006) Overexpression and diffuse expression pattern of IQGAP1 at invasion fronts are independent prognostic parameters in ovarian carcinomas. Cancer Lett 243: 120–127 [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI (2005) α-Catenin is a molecular switch that binds E-cadherin–β-catenin and regulates actin-filament assembly. Cell 123: 903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenmann KM, West RA, Hildebrand D, Kitchen SM, Peng J, Sigler R, Zhang J, Siminovitch KA, Alberts AS (2007) T cell responses in mammalian diaphanous-related formin mDia1 knockout mice. J Biol Chem 282: 25152–25158 [DOI] [PubMed] [Google Scholar]
- Eng K, Naqvi NI, Wong KC, Balasubramanian MK (1998) Rng2p, a protein required for cytokinesis in fission yeast, is a component of the actomyosin ring and the spindle pole body. Curr Biol 8: 611–621 [DOI] [PubMed] [Google Scholar]
- Faix J, Grosse R (2006) Staying in shape with formins. Dev Cell 10: 693–706 [DOI] [PubMed] [Google Scholar]
- Faix J, Weber I, Mintert U, Kohler J, Lottspeich F, Marriott G (2001) Recruitment of cortexillin into the cleavage furrow is controlled by Rac1 and IQGAP-related proteins. EMBO J 20: 3705–3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Fujii K, Nakamura T, Shoji I, Matsuura Y, Okawa K, Iwamatsu A, Kikuchi A, Kaibuchi K (1997) Regulation of cross-linking of actin filament by IQGAP1, a target for Cdc42. J Biol Chem 272: 29579–29583 [DOI] [PubMed] [Google Scholar]
- Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K (2002) Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell 109: 873–885 [DOI] [PubMed] [Google Scholar]
- Grohmanova K, Schlaepfer D, Hess D, Gutierrez P, Beck M, Kroschewski R (2004) Phosphorylation of IQGAP1 modulates its binding to Cdc42, revealing a new type of rho-GTPase regulator. J Biol Chem 279: 48495–48504 [DOI] [PubMed] [Google Scholar]
- Hart MJ, Callow MG, Souza B, Polakis P (1996) IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J 15: 2997–3005 [PMC free article] [PubMed] [Google Scholar]
- Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y (2004) Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J Cell Biol 166: 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HW, Li Z, Brown MD, Sacks DB (2007) IQGAP1 binds Rap1 and modulates its activity. J Biol Chem 282: 20752–20762 [DOI] [PubMed] [Google Scholar]
- Jia Z, Barbier L, Stuart H, Amrei M, Pelech S, Dennis JW, Metalnikov P, O'Donnel P, Nabi IR (2005) Tumor cell pseudopodial protrusions. J Biol Chem 280: 30564–30573 [DOI] [PubMed] [Google Scholar]
- Kholmanskikh SS, Koeller HB, Wynshaw-Boris A, Gomez T, Letourneau PC, Ross ME (2006) Calcium-dependent interaction of Lis1 with IQGAP1 and Cdc42 promotes neuronal motility. Nat Neurosci 9: 50–57 [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K (1996) Identification of IQGAP1 as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem 271: 23363–23367 [DOI] [PubMed] [Google Scholar]
- Kuroda S et al. (1998) Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell–cell adhesion. Science 281: 832–835 [DOI] [PubMed] [Google Scholar]
- Le Clainche C et al. (2007) IQGAP1 stimulates actin assembly through the N-WASP–Arp2/3 pathway. J Biol Chem 282: 426–435 [DOI] [PubMed] [Google Scholar]
- Li Z, Kim SH, Higgins JM, Brenner MB, Sacks DB (1999) IQGAP1 and calmodulin modulate E-cadherin function. J Biol Chem 274: 37885–37892 [DOI] [PubMed] [Google Scholar]
- Li Z, McNulty DE, Marler KJ, Lim L, Hall C, Annan AS, Sacks DB (2005) IQGAP1 promotes neurite outgrowth in a phosphorylation-dependent manner. J Biol Chem 280: 13871–13878 [DOI] [PubMed] [Google Scholar]
- Lippincott J, Li R (1998) Sequential assembly of myosin II, an IQGAP-like protein, and filamentous actin to a ring structure involved in budding yeast cytokinesis. J Cell Biol 140: 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM (1998) Cytokinesis: IQGAPs find a function. Curr Biol 8: R202–R205 [DOI] [PubMed] [Google Scholar]
- Mateer SC, McDaniel AE, Nicolas V, Habermacher GM, Lin MJ, Cromer DA, King ME, Bloom GS (2002) The mechanism for regulation of the F-actin binding activity of IQGAP1 by calcium/calmodulin. J Biol Chem 277: 12324–12333 [DOI] [PubMed] [Google Scholar]
- Mateer SC, Morris LE, Cromer DA, Bensenor LB, Bloom GS (2004) Actin filament binding by a monomeric IQGAP1 fragment with a single calponin homology domain. Cell Motil Cytoskeleton 58: 231–241 [DOI] [PubMed] [Google Scholar]
- May RC, Caron E, Hall A, Machesky LM (2000) Involvement of Arp2/3 complex in phagocytosis mediated by FcγR or CR3. Nat Cell Biol 2: 246–248 [DOI] [PubMed] [Google Scholar]
- McDonald KL et al. (2007) IQGAP1 and IGFBP2: valuable biomarkers for determining prognosis in glioma patients. J Neuropathol Exp Neurol 66: 405–417 [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Mabuchi I (2003) An IQGAP-like protein is involved in actin assembly together with Cdc42 in the sea urchin egg. Cell Motil Cytoskeleton 56: 207–218 [DOI] [PubMed] [Google Scholar]
- Noritake J, Fukata M, Sato K, Nakagawa M, Watanabe T, Izumi N, Wang S, Fukata Y, Kaibuchi K (2004) Positive role of IQGAP1, an effector of Rac1, in actin-meshwork formation at sites of cell–cell contact. Mol Biol Cell 15: 1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K (2005) IQGAP1: a key regulator of adhesion and migration. J Cell Sci 118: 2085–2092 [DOI] [PubMed] [Google Scholar]
- Osman MA, Cerione RA (1998) Iqg1p, a yeast homologue of the mammalian IQGAPs, mediates cdc42p effects on the actin cytoskeleton. J Cell Biol 142: 443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD (2007) Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct 36: 451–477 [DOI] [PubMed] [Google Scholar]
- Pruyne D, Evangelista M, Yang C, Bi E, Zigmond S, Bretscher A, Boone C (2002) Role of formins in actin assembly: nucleation and barbed-end association. Science 297: 612–615 [DOI] [PubMed] [Google Scholar]
- Ren JG, Li Z, Sacks DB (2007) IQGAP1 modulates activation of B-Raf. Proc Natl Acad Sci USA 104: 10465–10469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Li Z, Sacks DB (2005) IQGAP1 is a scaffold for mitogen-activated protein kinase signaling. Mol Cell Biol 25: 7940–7952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ (2002) ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol 4: 408–415 [DOI] [PubMed] [Google Scholar]
- Sakata D, Taniguchi H, Yasuda S, Adachi-Morishima A, Hamazaki Y, Nakayama R, Miki T, Minato N, Narumiya S (2007) Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med 204: 2031–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt VA, Scudder L, Devoe CE, Bernards A, Cupit LD, Bahou WF (2003) IQGAP2 functions as a GTP-dependent effector protein in thrombin-induced platelet cytoskeletal reorganization. Blood 101: 3021–3028 [DOI] [PubMed] [Google Scholar]
- Shannon KB, Li R (1999) The multiple roles of Cyk1p in the assembly and function of the actomyosin ring in budding yeast. Mol Biol Cell 10: 283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Shewan AM, Scott JA, Helwani FM, den Elzen NR, Miki H, Takenawa T, Yap AS (2004) Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J Biol Chem 279: 34062–34070 [DOI] [PubMed] [Google Scholar]
- Wang S et al. (2007) IQGAP3, a novel effector of Rac1 and Cdc42, regulates neurite outgrowth. J Cell Sci 120: 567–577 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K (2004) Interaction with IQGAP1 links APC to Rac1, Cdc42 and actin filaments during cell polarization and migration. Dev Cell 7: 871–883 [DOI] [PubMed] [Google Scholar]
- Weissbach L, Settleman J, Kalady MF, Snijders AJ, Murthy AE, Yan YX, Bernards A (1994) Identification of a human rasGAP-related protein containing calmodulin-binding motifs. J Biol Chem 269: 20517–20521 [PubMed] [Google Scholar]
- Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG (2004) EB1 and APC bind mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol 9: 820–830 [DOI] [PubMed] [Google Scholar]
- Wu J-Q, Pollard TD (2005) Counting cytokinesis proteins globally and locally in fission yeast. Science 310: 310–314 [DOI] [PubMed] [Google Scholar]
- Wu J-Q, Sirotkin V, Kovar DR, Lord M, Beltzner CC, Kuhn JR, Pollard TD (2006) Assembly of the cytokinetic contractile ring from a broad band of nodes in fission yeast. J Cell Biol 174: 391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Noguchi T, Mabuchi I (2003) Localization of two IQGAPs in cultured cells and early embryos of Xenopus laevis. Cell Motil Cytoskeleton 55: 36–50 [DOI] [PubMed] [Google Scholar]
- Yamashiro S, Abe H, Mabuchi I (2007) IQGAP2 is required for the cadherin-mediated cell-to-cell adhesion in Xenopus laevis embryos. Dev Biol 308: 485–493 [DOI] [PubMed] [Google Scholar]
- Zhang J et al. (1999) Antigen receptor-induced activation and cytoskeletal rearrangement are impaired in Wiskott–Aldrich syndrome protein-deficient lymphocytes. J Exp Med 190: 1329–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]