Abstract
Ultraviolet A (UVA) makes up more than 90% of incident terrestrial ultraviolet radiation. Unlike shorter wavelength UVB, which damages DNA directly, UVA is absorbed poorly by DNA and is therefore considered to be less hazardous. Organ transplant patients treated with the immunosuppressant azathioprine frequently develop skin cancer. Their DNA contains 6-thioguanine—a base analogue that generates DNA-damaging singlet oxygen (1O2) when exposed to UVA. Here, we show that this 1O2 damages proliferating cell nuclear antigen (PCNA), the homotrimeric DNA polymerase sliding clamp. It causes covalent oxidative crosslinking between the PCNA subunits through a histidine residue in the intersubunit domain. Crosslinking also occurs after treatment with higher—although still moderate—doses of UVA alone or with chemical oxidants. Chronic accumulation of oxidized proteins is linked to neurodegenerative disorders and ageing. Our findings identify oxidative damage to an important DNA replication and repair protein as a previously unrecognized hazard of acute oxidative stress.
Keywords: azathioprine, PCNA, reactive oxygen, skin cancer, thioguanine
Introduction
DNA lesions such as cyclobutane pyrimidine dimers and 6,4 pyrimidine photoproducts caused by the absorption of UVB (wavelength 290–320 nm) from sunlight, are important contributors to skin cancer (Friedberg et al, 2006). UVA (320–400 nm)—the predominant energy source in incident sunlight—is absorbed poorly by DNA and is therefore considered to be less harmful. The danger from UVA is mainly indirect; it produces thymine:thymine cyclobutane photoproducts in cellular DNA (Young et al, 1998; Courdavault et al, 2005), probably through photosensitized reactions involving non-DNA chromophores. UVA also generates reactive oxygen species (ROS)—principally singlet oxygen (1O2) (Cadet et al, 2005)—which damage proteins, lipids and nucleic acids. Although essential for normal cellular processes, excessive ROS are hazardous and cause oxidative stress. Persistent oxidative DNA damage is linked to mutation and to human cancer (Al-Tassan et al, 2002); the accumulation of oxidized proteins is associated with neurodegenerative disorders (Floyd & Hensley, 2002).
Unlike canonical DNA bases, some base analogues such as 6-thioguanine (6-TG), are strong UVA chromophores. 6-TG, 6-mercaptopurine and azathioprine (Aza) are anticancer and immunosuppressant thiopurines (Aarbakke et al, 1997). Aza, which is used extensively as an immunosuppressant following organ transplantation and is increasingly prescribed for inflammatory disorders (Lichtenstein et al, 2006), causes 6-TG to accumulate in patients' DNA (Warren et al, 1995; Cuffari et al, 1996; O'Donovan et al, 2005). Organ transplant patients have a high risk of skin cancer for which sunlight exposure is a cofactor. DNA 6-TG interacts with UVA to generate ROS, which oxidize the 6-TG to guanine-6-sulphonate (GSO3; O'Donovan et al, 2005), a powerful block to DNA polymerases in vitro (O'Donovan et al, 2005; Zhang et al, 2006). Here, we report that combined 6-TG–UVA also causes a new oxidative modification of the DNA replication and repair protein, proliferating cell nuclear antigen (PCNA).
Results
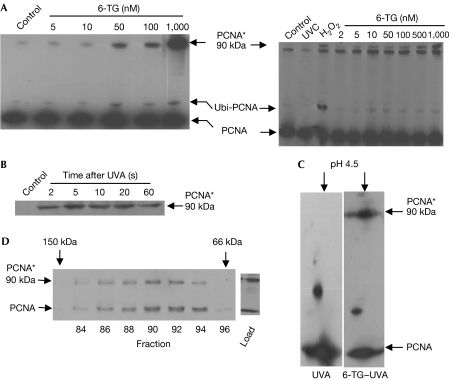
The main DNA 6-TG photoproduct GSO3, blocks primer extension by Klenow fragment DNA polymerase. In vivo, doses of UVA as low as 10 kJ/m2 cause a rapid inhibition of DNA synthesis in human cells containing DNA 6-TG (Zhang et al, 2006; data not shown). As Y family DNA polymerases bypass a replication-blocking template GSO3 in vitro (O'Donovan et al, 2005), we examined whether 6-TG–UVA treatment induced the cellular PCNA monoubiquitination associated with polymerase switching from replication to lesion bypass (Kannouche et al, 2004). Western blots showed an altered PCNA form that was consistent with monoubiquitination (Ubi-PCNA; Fig 1A). Strikingly, the same blots revealed a new high-molecular-weight PCNA species (PCNA*) of approximately 90 kDa (Fig 1A), which was particularly abundant in partly tetraploid CCF-CEM cells. It was also formed in HCT116 (Fig 1A), A2780 and Raji cells (supplementary Fig S1a online). Immunoprecipitated PCNA* was unreactive towards a ubiquitin antibody, eliminating polyubiquitination (data not shown). It was consistently observed as a discrete band rather than the smear that would be expected from DNA–protein crosslinking. PCNA* formed rapidly (Fig 1B) and persisted for several hours. It was not detectable after 3 kJ/m2 UVA or 6-TG treatment alone (Fig 1A and see below). The pI of PCNA* was approximately 4.5 and indistinguishable from that of a PCNA monomer (Fig 1C). On size-exclusion chromatography under nondenaturing conditions, PCNA* coeluted with native trimeric PCNA with a Ve corresponding to a globular protein of approximately 100 kDa (Fig 1D). We conclude that PCNA* is a covalent PCNA complex with a native mass and charge similar to that of an unmodified PCNA trimer. Thus, in addition to introducing potentially replication-blocking DNA lesions, 6-TG–UVA causes a rapid covalent modification of an important DNA replication and repair protein.
Figure 1.
Formation and characterization of a new high-molecular-weight PCNA species. (A) CCF-CEM (left panel) or HCT116 (right panel) cells were grown for 48 h in medium containing 6-TG at the concentrations indicated and irradiated with 3 kJ/m2 UVA. Both cell lines are mismatch repair defective and accumulate significant levels of DNA 6-TG (≥0.1% of DNA guanine) without toxic effect; control cells were unirradiated. HCT116 cells grown in the absence of 6-TG were also treated with H2O2 (0.03%, 2 min) or 10 J/m2 UVC. Chromatin-associated proteins extracted 6 h after irradiation, or immediately after H2O2 treatment, were analysed by western blotting with PC10 PCNA antibodies. The position of monoubiquitinated PCNA (Ubi-PCNA) is indicated. Immunoprecipitation experiments followed by western blotting with ubiquitin antibodies confirmed the presence of ubiquitin. The 90 kDa PCNA complex (PCNA*) is also shown. This complex migrated slightly more slowly than a nonspecific band that is more prominent in HCT116 than in CCF-CEM extracts. (B) Kinetics of PCNA* formation. HCT116 cells grown for 48 h in 1 μM 6-TG were irradiated with 1 kJ/m2 UVA at a high dose rate (0.5 kJ/m2/s). Extracts prepared at the time points indicated were analysed by western blotting with PC10. (C) PCNA* pI. Chromatin-associated proteins from CCF-CEM cells grown in the presence or absence of 6-TG (1 μM, 48 h), as indicated, and UVA irradiated (10 kJ/m2) were analysed by two-dimensional IEF/PAGE analysis followed by western blotting with PC10. (D) Size-exclusion chromatography. Chromatin-associated proteins extracted from CCF-CEM cells treated with 6-TG (1 μM, 48 h) and UVA (50 kJ/m2) were separated by chromatography on AcA44 precalibrated with alcohol dehydrogenase (150 kDa), BSA (66 kDa) and carbonic anhydrase (30 kDa). Fractions containing PCNA were identified by dot blot and analysed further by western blotting with PC10. 6-TG, 6-thioguanine; Control, unirradiated; IEF/PAGE, isoelectric focusing-polyacrylamide gel electrophoresis; PCNA, proliferating cell nuclear antigen; UVA, ultraviolet A.
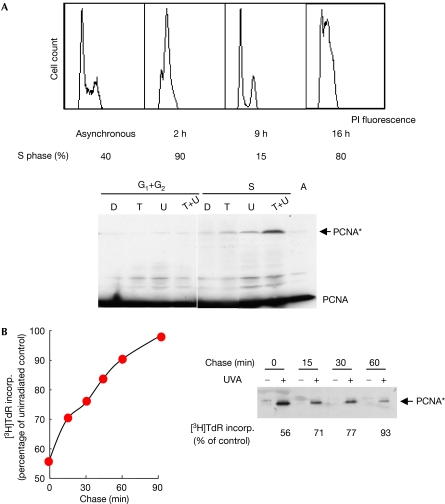
To examine whether PCNA* formed at active replication forks, where it is likely to be more hazardous, we compared the effect of 6-TG–UVA in S-phase and non-S-phase cells. Synchronized HCT116 cells released into S phase were allowed to incorporate 6-TG for 60 min. Fluorescence-activated cell sorting (FACS) analysis confirmed that more than 90% of the cells were in S phase during 6-TG treatment and that incorporated 6-TG did not affect subsequent cell-cycle progression. The first S phase was complete (≤15% S-phase cells) 9 h after 6-TG labelling. By 16 h, approximately 80% of 6-TG-treated and untreated cells had entered a second S phase (Fig 2A). Extracts were prepared from cells exposed to 10 kJ/m2 UVA at 9 h (G1+G2) or 16 h (S) after 6-TG treatment. Western blots indicated that despite similar levels of PCNA, irradiated G1+G2-phase cells contained little PCNA*, whereas the complex was readily detectable in cells irradiated in S phase (Fig 2A). These observations are consistent with the formation of PCNA* from PCNA engaged in replication; pulse–chase experiments confirmed this possibility. UVA irradiation of asynchronous cells, immediately after a 6-TG pulse, caused significant PCNA crosslinking (Fig 2B; supplementary Fig S1b online) and inhibited DNA synthesis. If irradiation was delayed to allow replication forks (with PCNA) to migrate away from the 6-TG-containing DNA, the amount of PCNA* declined together with the inhibition of DNA synthesis. DNA itself and active replisome proteins are the most likely targets of the highly unstable ROS generated from DNA 6-TG. A short stretch of DNA 6-TG close to the replisome is sufficient for a low dose of UVA to generate replication-blocking DNA lesions and PCNA*.
Figure 2.
Formation of a new high-molecular-weight PCNA species at active replication forks. (A) The 90 kDa PCNA complex (PCNA*) in non-S- compared with S-phase cells. Synchronized HCT116 cells were 6-TG labelled (60 min, 10 μM) 2 h after release from a double thymidine block. At this time ≥90% of cells were in S phase, as determined by FACS of propidium iodide (PI)-stained cells (upper panel). Cell-cycle distributions were assigned using Watson Pragmatic. Aliquots of 6-TG-treated cells were UVA irradiated (10 kJ/m2) either 9 h (when 85% of cells were in G1+G2 phase) or at 16 h (>80% S phase) after 6-TG treatment. Cell extracts were analysed by western blots probed with PC10 (lower panel); the positions of PCNA and PCNA* are indicated. Left: G1+G2 cells; right: S-phase cells. (B) 6-TG pulse–chase. Exponentially growing CCF-CEM cells were pulse labelled with 6-TG (10 μM, 15 min), followed by a return to normal growth medium without 6-TG. At the time points indicated, aliquots of cells were irradiated with UVA (10 kJ/m2). Immediately after irradiation, one sample of each aliquot was used to monitor DNA replication by [3H]thymidine incorporation, which is expressed as a percentage of that in unirradiated cells. The remaining cells were extracted and proteins analysed by western blotting with PC10. 6-TG, 6-thioguanine; A, asynchronous untreated cells; D, double thymidine block only; FACS, fluorescence-activated cell sorting; PCNA, proliferating cell nuclear antigen; T, 6-TG only; T+U, 6-TG+UVA; U, UVA only; UVA, ultraviolet A.
The UVA–6-TG interaction generates 1O2 (Zhang et al, 2006), a form of ROS implicated in protein crosslinking (Shen et al, 1996), and PCNA* was formed when chromatin proteins were treated with rose bengal and visible light—an acknowledged source of 1O2 (supplementary Fig S2 online). UVA also generates 1O2 in cells (Cadet et al, 2003) through absorption by non-DNA chromophores. Although the UVA doses (<10 kJ/m2) that we used did not generate PCNA* in the absence of DNA 6-TG, the complex was readily detected after higher—although still modest—doses of UVA alone (Fig 3A; supplementary Fig S1 online). We examined whether the trimeric structure of PCNA juxtaposes amino acids susceptible to crosslinking by 1O2. Inspection of the intersubunit region shows several candidate residues (Fig 3B). The proximity of His 153 and Lys 77 is particularly intriguing, as histidine–lysine crosslinking by 1O2 is highly favoured (Au & Madison, 2000). To investigate the possible involvement of His 153, we took advantage of the replacement of this otherwise highly conserved residue by glutamine in insects and Xenopus. Although PCNA* is detectable after 30 kJ/m2 UVA in human cells, it was not detected in extracts of SF9 (Spodoptera frugiperda) insect cells irradiated with up to 300 kJ/m2 UVA (Fig 3C); it was not possible to examine PCNA* formation by 6-TG–UVA in SF9 cells, which do not incorporate 6-TG. PCNA* was not formed following irradiation of replicating or nonreplicating Xenopus egg extracts (Fig 3C). This indicates that His 153 is required for photochemical PCNA crosslinking. PCNA can be crosslinked by aldehydes through the conserved Lys 110 residue (Wenz et al, 1998; Balajee & Geard, 2001; Naryzhny et al, 2005). Formaldehyde treatment of SF9 cells and Xenopus egg extracts generated high-molecular-weight PCNA complexes (Fig 3C), confirming the presence of trimeric PCNA. Formaldehyde and UVA (or UVA–6-TG) produced different PCNA complexes. This might reflect differences in the crosslinked amino acids (supplementary Fig S2b online). These findings implicate PCNA His 153 in the photochemical formation of intersubunit covalent bonds, probably through Lys 77.
Figure 3.
Photochemical formation of a new high-molecular-weight PCNA species requires a conserved histidine residue. (A) Crosslinking by UVA alone. Left panel: asynchronous A2780 cells grown in the presence or absence of 6-TG (0.2 μM, 48 h) were UVA irradiated as indicated; cell extracts were analysed by western blotting with PC10. Right panel: Raji cells synchronized by DMSO (2%, 48 h) were UVA (kJ/m2) or UVC (J/m2) irradiated as indicated in either G1 or S phase. PCNA was analysed by western blotting. G1 irradiated 7 h after release (>90% G0/G1 by PI staining and FACS analysis); S irradiated 20 h after release (>90% S phase). (B) Intersubunit region of human PCNA with alignment of conserved residues with reactive side chains. Lys 77 and Lys 110, and Arg 146 and Arg 149 are highly conserved; His 153 is replaced by glutamine in the insect Spodoptera frugiperda and in Xenopus laevis. Other potentially reactive intersubunit residues—Cys 81, Cys 148 and Met 116—are fully conserved. (C) Chemical and photochemical PCNA crosslinking. Human HCT116 or insect SF9 cells were treated for 15 min with formaldehyde or UVA irradiated. X. laevis egg extracts (Kubota & Takisawa, 1993) were induced to replicate by the addition of sperm nuclei. After 5 min, at which time point there was minimal replication (Replication−) as indicated by nucleus formation (Hoechst staining) and fluorescent dAMP from Cy3-ATP incorporation (dATP), or after 45 min, during the period of maximal DNA replication with significant dAMP incorporation into well-defined nuclei (Replication+), extracts were UVA irradiated. An aliquot of the same replication+extract was treated with formaldehyde. HCT116 cells treated with 6-TG (0.1 μM, 24 h) and irradiated with UVA (10 kJ/m2) were included as a control, C. Cell and egg extracts were analysed by western blotting (6% SDS–PAGE) using the PC10 antibody as probe. 6-TG, 6-thioguanine; DMSO, dimethyl sulphoxide; FACS, fluorescence-activated cell sorting; PCNA, proliferating cell nuclear antigen; PI, propidium iodide; UVA, ultraviolet A.
It has been suggested that aldehydes generate a covalent circular PCNA trimer of approximately 86 kDa (Wenz et al, 1998; Balajee & Geard, 2001; Naryzhny et al, 2005); the size and pI of PCNA* are consistent with this possibility. In preliminary analyses, immunoprecipitated PCNA* co-purified with polypeptides of approximately 83 kDa on SDS–polyacrylamide gel electrophoresis (supplementary Table 1 online). No co-purifying protein of 54 kDa was identified, indicating that PCNA* does not comprise a 29 kDa PCNA monomer and a non-PCNA protein. The observation is consistent with covalent crosslinking between the three PCNA subunits; however, non-circular PCNA multimers are not excluded by our findings.
Discussion
Ultraviolet in sunlight, of which more than 90% is UVA, is a significant risk factor for skin cancer in organ transplant patients (Euvrard et al, 2003). Aza immunosuppression produces DNA 6-TG—a source of 1O2 on UVA exposure. Photochemical PCNA crosslinking at replication forks is a previously unsuspected sunlight-related hazard for these patients. Higher, but nevertheless still moderate, doses of UVA that generate ROS through cellular photosensitizers also form PCNA*. PCNA might be particularly susceptible to oxidative stress. In agreement with this possibility, the chemical oxidants H2O2 and KBrO3 also crosslinked PCNA (supplementary Fig 2c online; Fig 1A).
PCNA improves the speed and processivity of DNA polymerases. It provides a scaffold for DNA–protein interactions in cellular signalling and DNA replication. It also participates in DNA mismatch repair (reviewed by Jiricny, 2006), and in nucleotide and base excision repair (reviewed by Friedberg et al, 2006). The extent to which the formation of PCNA* compromises these important functions remains to be determined. It might have a bearing on curious and unexplained differences in the efficiency of thymine:thymine cyclobutane dimer removal. These DNA photoproducts are reportedly excised less efficiently by nucleotide excision repair if they are induced by UVA compared with UVB (Courdavault et al, 2005). It is tempting to speculate that oxidative damage to PCNA or other repair proteins might contribute to this inefficiency. PCNA* is an oxidation-related modification that depends on a reactive histidine in the intersubunit domain. A highly favoured reaction might be compatible with a rapid joining of all PCNA subunits to form the proposed covalent circular trimer (Wenz et al, 1998; Balajee & Geard, 2001; Naryzhny et al, 2005). PCNA* is easily detected by its significantly changed electrophoretic mobility. It seems unlikely that this is the only form of protein damage caused by 1O2 generated in DNA, and PCNA* might be a marker for general, perhaps more subtle, oxidative modifications of replication proteins. Chronic protein oxidation is implicated in ageing and in some neurological disorders (Floyd & Hensley, 2002). The relationships between oxidative DNA damage—the formation, repair and biological impact of oxidized DNA bases—and cancer have been extensively studied. Our findings indicate that examination of the impact of oxidative stress on the efficiency of DNA replication and repair proteins might also be warranted.
Methods
Cells and cell culture. A2780 SC5 ovarian carcinoma cells (Zhang et al, 2006) and HCT116 colorectal carcinoma cells were grown in DMEM, Raji Burkitt's lymphoma and CCF-CEM leukaemia cells in RPMI, and SF9 cells in Grace's medium, all supplemented with 10% FCS. HCT116 cells were synchronized by double thymidine block, and Raji cells by 2% dimethyl sulphoxide (Fiore et al, 2002). Replication was measured by [3H]TdR labelling (1 μCi/ml, 15 min, 1 × 106 cells), followed by the determination of trichloroacetic acid-insoluble radioactivity.
Xenopus egg extracts (Kubota & Takisawa, 1993) were induced to replicate by the addition of sperm nuclei.
Chemicals and reagents. Chemicals were obtained from Sigma-Aldrich (Poole, UK). 6-TG in 0.1 N NaOH was diluted in culture medium before cell treatment. PCNA (PC10) and ubiquitin (P4D1) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Irradiation. UVA from a UVH 253 lamp (UVLight Technology, Birmingham, UK), fitted with a black glass filter to remove wavelengths less than 320 nm, was routinely delivered at a dose rate of 0.1 kJ/m2/s. UVC (254 nm) was delivered from a germicidal lamp with a dose rate of 1 J/m2/s.
Chemical treatment. Cells were treated with formaldehyde in PBS. For H2O2 and KBrO3 treatments, cells were in growth medium.
Cell extracts and analysis. A total of 1 × 106 cells were lysed in 100 μl cold 1% NP-40, 10 mM NaF, 1 mM Na3VO4, 1 mM Na4P2O7 plus protease inhibitor cocktail (Roche Diagnostics, Lewes, UK). After 1 h incubation on ice, insoluble material was removed by centrifugation (30 min, 11,000g) and discarded.
For chromatin-enriched extracts (Balajee & Geard, 2001), cells were suspended in cold 10 mM Tris–HCl (pH 7.5), 2.5 mM MgCl2, 0.5% NP-40 plus protease inhibitors. After 8 min incubation on ice, extracts were centrifuged (750g, 5 min) and the pellet was resuspended in chromatin extraction buffer (25 mM Na4P2O7 pH 7.4, 0.5 M NaCl, 1 mM EDTA, 0.5% Triton X-100, 10% glycerol, 5 mM MgCl2 and protease inhibitors). After a further 20 min incubation on ice, the extracts were centrifuged (5 min, 12,000g) and the supernatant was retained.
Proteins were resolved on 8% SDS gels for western blotting. Complexes were detected by using enhanced chemiluminescent substrate and visualized on Hyperfilm (Amersham Pharmacia, Little Chalfont, UK). Protein size estimates are based on Prestained Low Molecular Weight Range Markers (Bio-Rad, Hemel Hempstead, UK).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figure S1 and Legends
Supplementary Figure S2 and Legends
Supplementary Table 1
Acknowledgments
We thank M. Singleton for advice on PCNA structure, A. Errico for help with the Xenopus egg experiments, and D. Davies, Cancer Research UK FACS Laboratory, for FACS analysis. The work was partly supported by a Royal Society KC Wong fellowship (X.Z.), the Association for International Cancer Research (C.M.P.) and the Spanish Ministerio de Educacion y Ciencia (B.M.).
References
- Aarbakke J, Janka-Schaub G, Elion GB (1997) Thiopurine biology and pharmacology. Trends Pharmacol Sci 18: 3–8 [DOI] [PubMed] [Google Scholar]
- Al-Tassan N et al. (2002) Inherited variants of MYH associated with somatic G:C → T:A mutations in colorectal tumors. Nat Gen 30: 227–232 [DOI] [PubMed] [Google Scholar]
- Au V, Madison SA (2000) Effects of singlet oxygen on the extracellular matrix protein collagen: oxidation of the collagen crosslink histidinohydroxylysinonorleucine and histidine. Arch Biochem Biophys 384: 133–142 [DOI] [PubMed] [Google Scholar]
- Balajee AS, Geard CR (2001) Chromatin-bound PCNA complex formation triggered by DNA damage occurs independent of the ATM gene product in human cells. Nucleic Acids Res 29: 1341–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J, Douki T, Gasparutto D, Ravanat J-L (2003) Oxidative damage to DNA: formation, measurement and biochemical features. Mutat Res 531: 5–23 [DOI] [PubMed] [Google Scholar]
- Cadet J, Sage E, Douki T (2005) Ultraviolet radiation-mediated damage to cellular DNA. Mutat Res 571: 3–17 [DOI] [PubMed] [Google Scholar]
- Courdavault S, Baudouin C, Charveron M, Canguilhem B, Favier A, Cadet J, Douki T (2005) Repair of the three main types of bipyrimidine DNA photoproducts in human keratinocytes exposed to UVB and UVA radiations. DNA Repair 4: 836–844 [DOI] [PubMed] [Google Scholar]
- Cuffari C, Seidmen EG, Latour S, Theoret Y (1996) Quantitation of 6-thioguanine in preipheral blood leukocyte DNA in Crohn's disease patients on maintenance 6-mercaptopurine therapy. Can J Physiol Pharmacol 74: 580–585 [PubMed] [Google Scholar]
- Euvrard S, Kanitakis J, Claudy A (2003) Skin cancers after organ transplantation. N Engl J Med 348: 1681–1691 [DOI] [PubMed] [Google Scholar]
- Fiore M, Zanier R, Degrassi F (2002) Reversible G1 arrest by dimethyl sulfoxide as a new method to synchronize Chinese hamster cells. Mutagenesis 17: 419–424 [DOI] [PubMed] [Google Scholar]
- Floyd RA, Hensley K (2002) Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging 23: 795–807 [DOI] [PubMed] [Google Scholar]
- Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T (2006) DNA Repair and Mutagenesis. Washington, USA: ASM [Google Scholar]
- Jiricny J (2006) The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 7: 335–346 [DOI] [PubMed] [Google Scholar]
- Kannouche PL, Wing J, Lehmann AR (2004) Interaction of human DNA polymerase η with monoubiquitinated PCNA: a possible mechanism for the polymerase switch in response to DNA damage. Mol Cell 14: 491–500 [DOI] [PubMed] [Google Scholar]
- Kubota Y, Takisawa H (1993) Determination of initiation of DNA replication before and after nuclear formation in Xenopus egg cell free extracts. J Cell Biol 123: 1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein GR, Abreu MT, Cohen R, Tremaine W (2006) American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 130: 940–987 [DOI] [PubMed] [Google Scholar]
- Naryzhny SN, Zhao H, Lee H (2005) Proliferating cell nuclear antigen (PCNA) may function as a double homotrimer complex in the mammalian cell. J Biol Chem 280: 13888–13894 [DOI] [PubMed] [Google Scholar]
- O'Donovan P, Perrett C, Zhang X, Montaner B, Xu Y-Z, Harwood CA, McGregor JM, Walker SL, Hanaoka F, Karran P (2005) Azathioprine and UVA light generate mutagenic oxidative DNA damage. Science 309: 1871–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HR, Spikes JD, Kopecekova P, Kopecek J (1996) Photodynamic crosslinking of proteins. II. Photocrosslinking of a model protein-ribonuclease A. J Photochem Photobiol B 35: 213–219 [DOI] [PubMed] [Google Scholar]
- Warren DJ, Andersen A, Slordal L (1995) Quantitation of 6-thioguanine residues in peripheral blood leukocyte DNA obtained from patients receiving 6-mercaptopurine-based maintenance therapy. Cancer Res 55: 1670–1674 [PubMed] [Google Scholar]
- Wenz F, Azzam EI, Little JB (1998) The response of proliferating cell nuclear antigen to ionizing radiation in human lymphoblastoid cells is dependent on p53. Radiat Res 149: 32–40 [PubMed] [Google Scholar]
- Young AR, Potten CS, Nikaido O, Parsons PG, Boenders J, Ramsden JM, Chadwick CA (1998) Human melanocytes and keratinocytes exposed to UVB or UVA in vivo show comparable levels of thymine dimers. J Invest Dermatol 111: 936–940 [DOI] [PubMed] [Google Scholar]
- Zhang X, Jeffs G, Ren X, O'Donovan P, Montaner B, Perrett CM, Karran P, Xu Y-Z (2006) Novel DNA lesions generated by the interaction between therapeutic thiopurines and UVA light. DNA Repair 6: 344–354 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1 and Legends
Supplementary Figure S2 and Legends
Supplementary Table 1