Abstract
Cardiac autonomic neuropathy associated with diabetes can cause silent myocardial ischemia and may influence the way that patients perceive symptoms of acute coronary syndromes (ACS). The purpose of this study was to examine symptoms of ACS in patients with and without diabetes while controlling for length of time with diabetes. A convenience sample of 256 patients from two large medical centers in the Midwest participated. Patients with diabetes comprised 33.2% of the sample and reported significantly less chest pain and more unusual fatigue. Patients with diabetes of longer duration (10 or more years) reported more difficulty breathing than did patients with diabetes of shorter duration (fewer than 10 years). Older patients with the same diabetes status also reported less chest pain. For older patients and for patients with diabetes, lack of chest pain during ACS could delay treatment and is thus a concern.
Keywords: diabetes mellitus, acute coronary syndromes, myocardial ischemia, symptoms
Diabetes is the fifth-leading cause of death in the United States. Over 65% of these deaths are attributed to cardiovascular disease (American Diabetes Association, n.d.). Diabetes confers a two- to fivefold greater risk of cardiac mortality for women and a threefold greater risk of cardiac mortality for men as compared to age and sex-matched persons without diabetes (Blendea, McFarlane, Isenovic, Gick, & Sowers, 2003). Increased mortality has been linked to a greater prevalence of silent ischemia associated with diabetes (Chico, Tomas, & Novials, 2005). It has been suggested that one mechanism of silent ischemia is cardiac autonomic neuropathy (CAN), which is a form of autonomic neuropathy involving damage to autonomic fibers, innervating blood vessels and the heart (Manzella & Paolisso, 2005). This damage may affect the afferent pathways that carry pain messages from the myocardium to the cerebral cortex, leading to diminished or absent chest pain during acute coronary syndromes (ACS; Langer, Freeman, Josse, Steiner, & Armstrong, 1991). Twenty-two percent of persons with type 2 diabetes have CAN, and silent ischemia occurs more frequently with diabetes in the presence of CAN (38%) as opposed to the absence of CAN (5%; Vinik, Freeman, & Erbas, 2003). The effects of CAN are not trivial. Manzella and Paolisso (2005) reported that mortality rates are 53% for those with CAN 5 years after diagnosis.
Diabetes, CAN, and Silent Myocardial Ischemia
The consequences of silent ischemia can be grave because lack of symptoms and lack of symptom recognition can lead to delay in seeking immediate medical assistance during ACS and can be a factor in sudden cardiac death. There is limited research examining the cardiac symptoms reported by patients with diabetes who present with ACS. Funk, Naum, Milner, and Chyun (2001) reported no difference in type of cardiac symptoms experienced by persons with and without diabetes who presented to the emergency department. However, patients with diabetes did experience more severe symptoms. Thuresson, Jarlov, Lindahl, Svensson, Zedigh, and Herlitz (2005) also found no differences in cardiac symptoms by diabetes status; however, only those experiencing chest pain or discomfort were included in the study.
Other studies have found differences in cardiac symptoms, depending on diabetes status. Canto et al. (2000) noted that a higher proportion of patients with diabetes who were admitted to the hospital with myocardial infarction (MI) had no chest pain. In another study, patients with diabetes experienced less nausea, more hyperventilation, and less aching and squeezing-type pain during an episode of unstable angina (DeVon, Penckofer, & Zerwic, 2005). Brieger et al. (2004) found that atypical symptoms of ACS—including dyspnea, diaphoresis, syncope, and nausea and vomiting—occurred more frequently in patients with diabetes in the absence of chest pain. Mayer and Rosenfeld (2006) examined the role of diabetes in symptom interpretation in a sample of women with MI. Shortness of breath was a common presenting symptom for the women with diabetes. It is not known if any of these symptom experiences can be attributed to CAN, because CAN was not measured in any of these investigations.
Recognition of the symptoms of ACS may also be complicated by the chronic symptoms associated with diabetes and complications of diabetes. Hypoglycemia-induced autonomic stimulation has resulted in palpitations, tremor, and anxiety (adrenergic stimulation) and sweating (cholinergic stimulation). Symptoms associated with glycemic fluctuations—such as stomach pain, shortness of breath, heartburn, and sweating—are similar to symptoms experienced during ACS (Bulpitt, Palmer, Battersby, & Fletcher, 1998; Malecki et al., 2000). Diabetes complicated by heart failure is associated with chest discomfort, shortness of breath, fatigue, nausea, weakness, and dizziness (Hunt et al., 2005). These similarities in symptoms may challenge and confuse patients as they attempt to label their symptoms and decide on a course of action.
No studies were found that established a link between duration of diabetes and symptoms experienced during ACS. However, data from the Diabetes Control and Complications Trial / Epidemiology of Diabetes Interventions and Complications study suggest a causal role between hyperglycemia and the development of microvascular disease over time (Diabetes Control and Complications Trial Research Group, 1993; Nathan et al., 2005). Participants in the “diabetes control and complications trial” arm of the study were followed for a mean of 6.5 years, and participants in the “epidemiology of diabetes interventions and complications” arm were followed for a mean of 17 years. These findings imply that changes in symptoms of ACS associated with microvascular changes may not be manifested for many years. Additionally, participants in the studies included only those with type 1 diabetes; as such, determining whether these findings apply to patients with type 2 diabetes will require further study.
The aims of this study were conceptually framed on the known physiological differences attributed to CAN and how these differences may be manifested in the symptom experience between persons with and without diabetes. The purpose of this study was to determine if there were differences in the symptoms of ACS between patients with and without diabetes. Specific aims were to identify differences in symptom type, location of pain or discomfort, and quality of pain or discomfort after controlling for duration of diabetes, age, sex, and functional status. Based on the literature review, our hypothesis was that chest pain would be less prevalent in a sample of patients with diabetes who were admitted through the emergency department and hospitalized for ACS.
Method
Design, Sample, and Setting
A convenience sample of 272 patients hospitalized with an admitting diagnosis of ACS was recruited for this descriptive cross-sectional study. Participants included 122 women and 150 men admitted to the cardiac step-down units of two regional medical centers in the Midwest, both of which serve as referral centers from rural and suburban hospitals. Data collection sites were chosen to recruit a heterogeneous sample representative of the local population and to recruit adequate numbers of minorities. Patients were eligible for study if they were admitted with a diagnosis of ACS, fluent in English, at least 21 years of age, admitted through the emergency department at least 12 hours before interview, free of sedation, pain-free, and had adequate cognitive capacity.
Patients were excluded if they had documentation of prior heart failure, elevated brain natriuretic peptide, or any history of cocaine use. We thought that the symptom experience might vary for those with a history of heart failure because many of the symptoms of which, including dyspnea and unusual fatigue, are similar to the acute symptoms of ACS. Patients with a history of cocaine use were excluded because ischemia is more likely to be precipitated by vasoconstriction, tachycardia, systemic hypertension, and increased myocardial oxygen consumption following cocaine ingestion when compared to the more common and chronic obstructive processes associated with coronary heart disease (CHD; Hollander, 2003). These exclusion criteria were adopted to control for threats to the internal validity of the findings—specifically, that the results reflect the true symptoms of ACS and not some other acute or chronic condition. Medical records were reviewed, and final diagnoses were recorded following discharge. Sixteen patients had a noncardiac discharge diagnosis (ICD-9 code other than 410 or 411) and were excluded from analyses, resulting in a final sample of 256.
Measures
The Symptoms of Acute Coronary Syndromes Inventory (SACSI), Canadian Cardiovascular Society (CCS) classification of angina, and a medical record review form were used to collect data.
SACSI
The SACSI was developed for a previous study on unstable angina (DeVon & Zerwic, 2003). The design of SACSI was based on an extensive review of the literature. Symptoms of ACS are a multidimensional construct; therefore, the SACSI consists of three sections, describing the type, location, and quality of symptoms. The first section includes 20 symptoms identified in the literature for patients with acute chest pain, MI, or unstable angina (Dempsey, Dracup, & Moser, 1995; McSweeney & Crane, 2000; Zerwic, 1998). Symptoms are measured on a 5-point scale: Patients indicate that they did not experience the symptom (0), or they rate the severity of each symptom as mild (1), moderate (2), severe (3), or very severe (4).
The second section includes 14 anatomic locations where pain or discomfort is experienced, and the final section contains 14 descriptors of the quality of the pain or discomfort. All items related to location and descriptors of discomfort are measured dichotomously (yes/no). Finally, patients were asked to describe the severity of their chest pain on a scale of 0–10. They were also asked to rate their worst symptom on a scale of 0–10, because chest pain was not always identified as the worst presenting symptom.
Content validity was established by published cardiovascular experts on two occasions (DeVon & Zerwic, 2003). Those items rated not relevant were removed from the instrument. The original content validity index for the entire instrument was .88 (p < .05). Prior to the start of this study the tool was again reviewed by five content experts and the computed Content Validity Index was .94 (p < .05).
Support for construct validity of the tool as a comprehensive measure of the symptoms of ACS was evident when patients did not add symptoms even though they were given the opportunity to do so during interview. Internal consistency reliability for the current sample was adequate (α = .81).
CCS classification of angina
The CCS classification of angina was used to assess patients’ levels of physical function (Campeau, 1976). We hypothesized that those patients in a higher CCS class would report more severe symptoms as well as more symptoms attributed to chronic angina or other comorbid conditions. The reliability and validity of the classification system has been established in a number of patient populations (Campeau, 1976).
Procedure
Data were collected over a 25-month period from July 2003 to August 2005, following approval from each institutional review board. Eligible patients were approached by their primary nurse and asked permission for their names, to be given to the researchers in accordance with Health Insurance Portability and Accountability Act guidelines. A member of the research team then approached patients and explained the study. Those who agreed were invited to participate, and written informed consent was obtained. Cognitive function was established during the consent process. Cognitive ability was deemed adequate if the participant was able to read and understand the consent form and explain the purpose of the study to the researcher. The investigators recorded all responses because of the acute nature of the patient’s illness. Confidentiality was ensured, given that each interview was conducted in the patient’s private hospital room.
A total of 282 names were supplied by hospital staff. Ten patients, six women and four men, declined to participate because of fatigue, lack of interest, or refusal to sign the consent form. Six of the 10 were African American, and ages ranged from 40 to 85. Sixteen patients were excluded based on a non-cardiac discharge diagnosis, resulting in the final sample of 256.
To reduce threats to internal validity caused by inclusion of prodromal symptoms, symptoms experienced during a prior episode of ACS, or symptoms occurring as a result of another illness, patients were asked to report only the symptoms that brought them to the emergency department on this admission. Diagnoses, diagnostic test results, risk factors, and results of physical exams were abstracted from medical records following completion of the interview.
Data Analyses
Data were coded and entered into SPSS 13.0. Descriptive statistics were computed using chi-square for categorical data and t tests for interval-level data. All tests were two-sided, and statistical significance was set at p < .05, with one exception; because of Bonferroni adjustment, p ≤ .0167 was necessary to achieve statistical significance for tests of symptoms by diabetes duration. The 20 symptom variables were recoded to dichotomous variables (yes/no). We believed that the presence or absence of symptoms was more important in the population of patients with diabetes who may be experiencing peripheral neuropathies or cardiac autonomic neuropathies. For those patients with diminished sensations, making the fine gradations of symptom severity could be difficult. Logistic regression analyses were used for each of the 20 dichotomized symptom variables. Diabetes was automatically included in the model, and a combination of forward and stepwise model selection was used to find the best-fitting model from the following covariates: age, sex, and CCS class. All two-way interactions for initially significant main effects were included in the model.
Duration of diabetes was examined because we hypothesized that the symptom experience would vary by time since diabetes was diagnosed. Patients were grouped into two categories: those who reported having diabetes for less than 10 years (n = 38) and those who had diabetes 10 years or more (n = 39). Eight patients could not recall how long they had had diabetes, and the time since diagnosis was not recorded in the medical record. These cutoff points were chosen because 10 years represented the 50th percentile of patients as well as the median duration. Logistic regression was also used to determine what factors predicted the presence of chest pain, given that this is the key symptom of ACS. Diabetes status and the covariates listed above were entered into the model.
Findings
Demographic and Diagnostic Characteristics
Of the total sample, 33.2% had diabetes (n = 85). Two patients had type 1 diabetes, and 83 had type 2 diabetes. There was no difference in mean age between groups; however, the patients without diabetes covered a larger age range (Table 1). Duration of diabetes ranged from 1 to 45 years for those with type 2 diabetes and from 45 to 70 years for the 2 patients with type 1 diabetes. Therefore, median duration of diabetes was examined. Because the median duration was only 10 years and there was no theoretical basis for believing that the symptom experience during ACS varies between patients with type 1 or type 2 diabetes, the 2 patients with type 1 diabetes were included in the analyses. Women had diabetes for a longer period as compared with men (16.4 years vs. 11.3 years), but the difference was not significant (p = .09). There were no significant differences in type of ACS, levels of education, marital status, or ethnicity between those patients with and without diabetes.
Table 1.
Demographic and Diagnostic Characteristics of Patients With and Without Diabetes
| Characteristics | Diabetes (n = 85) | No diabetes (n = 171) | p |
|---|---|---|---|
| Age (years) | |||
| M (SD) | 65.5 (12.4) | 63.9 (14.2) | .21 |
| Range | 39–87 | 24–97 | |
| n (%) | n (%) | ||
|
| |||
| Gender | |||
| Female | 43 (51) | 69 (40) | .08 |
| Male | 42 (49) | 102 (60) | |
| Race/ethnicity | |||
| Black (n = 51) | 23 (27) | 28 (16) | .06 |
| White (Non-Hispanic) (n = 191) | 56 (66) | 135 (79) | |
| Hispanic (n = 8) | 5 (6) | 3 (2) | |
| Asian / Pacific Islander (n = 3) | 1 (1) | 2 (1) | |
| Native American (n = 3) | 0 | 3 (2) | |
| Incomea | .02 | ||
| ≤ $20,000 (n = 84) | 29 (35) | 55 (32) | |
| $20,001–$50,000 (n = 81) | 32 (40) | 59 (35) | |
| > $50,000 (n = 47) | 10 (12) | 37 (22) | |
| Type of acute coronary syndromes | |||
| Unstable angina (n = 88) | 35 (40) | 53 (31) | .07 |
| Non-ST elevation myocardial infarction (n = 84) | 30 (36) | 54 (32) | |
| ST elevation myocardial infarction (n = 84) | 20 (24) | 64 (37) | |
Indicates missing data.
Baseline Characteristics
Body mass index data were calculated and recoded into five categories based on American Heart Association definitions (n.d.): underweight, normal weight, overweight, obese, and extreme obesity. Patients with diabetes were more likely to be obese and extremely obese and less likely to be normal weight (Table 2). As expected, those with diabetes were more likely to have a history of CHD (p = .001), MI (p = .008), angina (p = .007), angiogram (p < .001), and/or percutaneous coronary intervention (p = .004). Patients with diabetes had more limitations in physical functioning, as measured by CCS class. Those with diabetes were more likely to be in Class 3 or Class 4, representing marked limitations to ordinary activity or pain at rest (χ2 = 16.06, p = .003).
Table 2.
Baseline Characteristics of Patients With and Without Diabetes
| Characteristics | Diabetes (n = 85) n (%) | No diabetes (n = 171) n (%) | P |
|---|---|---|---|
| Body mass indexa | |||
| Underweight (< 18.5) | 0 (0.0) | 3 (1.8) | |
| Normal weight (18.5–24.9) | 10 (8.4) | 46 (26.9) | |
| Overweight (25.0–29.9) | 27 (32.1) | 54 (31.6) | |
| Obese (30.0–39.9) | 37 (44.0) | 59 (34.5) | |
| Morbidly obese (≥ 40.0) | 10 (8.4) | 9 (5.3) | .018 |
| Health history | |||
| Prior diagnosis of CHD | 53 (62.4) | 68 (39.8) | .001 |
| Prior myocardial infarction | 38 (44.7) | 48 (28.1) | .008 |
| Prior angina | 51 (60.0) | 72 (42.1) | .007 |
| Prior angiogram | 61 (71.8) | 78 (45.6) | < .001 |
| Prior angioplasty/stent | 43 (50.6) | 55 (32.2) | .004 |
| Prior or current smoker | 18 (21.2) | 51 (29.8) | .142 |
| CCS classa | |||
| Class 1 | 37 (43.5) | 104 (60.8) | |
| Class 2 | 17 (20.0) | 42 (24.6) | |
| Class 3 | 22 (25.9) | 17 (9.9) | |
| Class 4 | 8 (9.4) | 7 (4.1) | .003 |
Note: CHD = coronary heart disease; CCS = Canadian Cardiovascular Society.
Denotes missing data.
Symptom Report by Diabetes Status
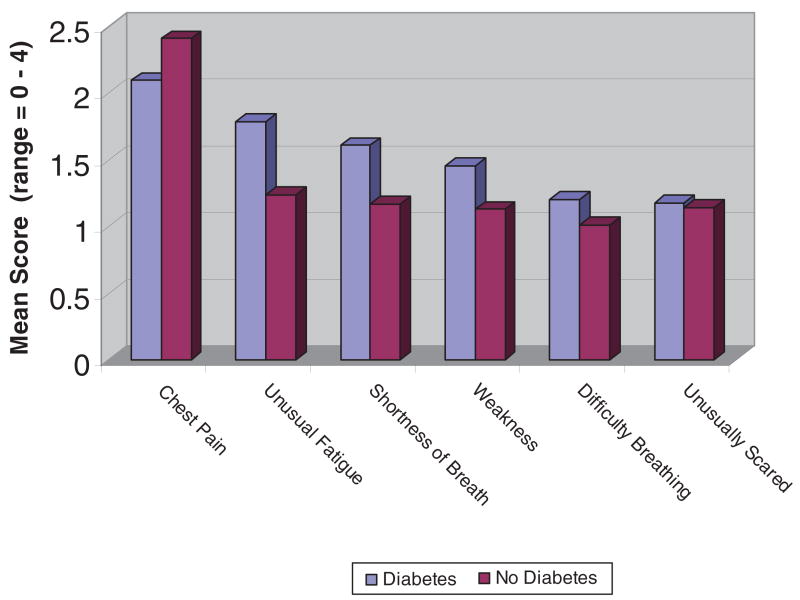
Patients were asked to report on the presence or absence of 20 symptoms, along with the severity of the symptoms following admission for ACS. The most frequently reported symptoms reported by patients with diabetes were chest pain, unusual fatigue, shortness of breath, and weakness. This matches the order of symptoms reported by the total sample. Patients without diabetes most frequently recounted chest pain, shortness of breath, unusual fatigue, and being unusually scared.
Figure 1 illustrates the severity of all symptoms reported by more than 50% of participants (shown by diabetes status). Mean severity of chest pain was 6.26 (SD = 3.24) for those with diabetes and 6.63 (SD = 3.17) for those without diabetes (p = .39). Mean scores for severity of the worst symptom was 7.26 (SD = 2.92) for the diabetes group versus 7.34 (SD = 2.67) for the group without diabetes (p = .82). Most important, patients with diabetes were nearly half as likely to experience chest pain (odds ratio [OR] = 0.46, confidence interval [CI] = 0.22–0.94, p = .034) and more than twice as likely to experience unusual fatigue as a patient of the same age and sex without diabetes (OR = 2.27, CI = 1.26–4.08, p = .006). There were no significant differences between groups on other symptoms.
Figure 1. Severity of Frequently Reported Symptoms by Diabetes Status.
Note: Range = 0–4.
Patients who had diabetes for 10 years or longer were more likely to experience difficulty breathing in comparison to patients who had diabetes for less than 10 years (OR = 5.84, CI = 1.70–20.01, p < .001) and patients without diabetes (OR = 2.94, CI = 1.07–8.05, p = .01; see Table 3). Finally, because chest pain is the most frequently reported symptom of ACS, logistic regression was used to examine predictors of chest pain. Age and diabetes were consistently significant, regardless of model selection methods used. Results indicate that both diabetes (OR = 0.46, CI = 0.22–0.94, p = .034) and older age (OR = 0.96, CI = 0.93–0.99, p = .005) are associated with the absence of chest pain. Bootstrapping techniques, in which random subsamples of the data are drawn and logistic regression is run on the sub-sample, were run to validate the model.
Table 3.
Symptom Differences and Duration of Diabetes
| Symptom | OR | SE | Lower CI | Upper CI | p |
|---|---|---|---|---|---|
| Difficulty breathing | |||||
| Short term vs. no diabetes | 0.50 | 0.19 | 0.21 | 1.22 | 0.06 |
| Long term vs. no diabetes | 2.94 | 1.24 | 1.07 | 8.05 | 0.01 |
| Long- vs. short-term diabetes | 5.84 | 3.01 | 1.70 | 20.01 | 0.00 |
| Hyperventilating | |||||
| Short-term vs. no diabetes | 0.85 | 0.38 | 0.30 | 2.44 | 0.72 |
| Long-term vs. no diabetes | 2.48 | 0.97 | 0.97 | 6.30 | 0.02 |
| Long- vs. short-term diabetes | 2.90 | 1.52 | 0.82 | 10.20 | 0.04 |
| Indigestion | |||||
| Short-term vs. no diabetes | 1.93 | 0.72 | 0.79 | 4.74 | 0.08 |
| Long-term vs. no diabetes | 0.54 | 0.25 | 0.17 | 1.65 | 0.19 |
| Long- vs. short-term diabetes | 0.28 | 0.15 | 0.08 | 1.04 | 0.02 |
| Unusual fatigue | |||||
| Short-term vs. no diabetes | 1.46 | 0.54 | 0.61 | 3.51 | 0.30 |
| Long-term vs. no diabetes | 2.73 | 1.20 | 0.96 | 7.79 | 0.02 |
| Long- vs. short-term diabetes | 1.87 | 0.98 | 0.53 | 6.58 | 0.24 |
Note: OR = odds ratio; CI = confidence interval.
p ≤ .0167.
Discussion
This study has several strengths: First, characteristics of the sample—including age, sex, and diagnoses—were consistent with the typical patient who presents with ACS. Second, a representative sample of African Americans, who are known to be at greater risk for diabetes, were recruited. Third, a majority (92%) of the patients with diabetes were overweight or obese, which is associated with type 2 diabetes. Fourth, the greater incidence of CHD, MI, angina, interventional procedures, and higher CCS class would be expected in patients with diabetes, given that diabetes is a CHD equivalent.
The most common symptoms reported by patients with diabetes were chest pain, unusual fatigue, shortness of breath, and weakness. These are consistent with prior findings and are of concern for those with silent ischemia because unusual fatigue, shortness of breath, and weakness are vague symptoms that are not likely to be interpreted as being serious (DeVon et al., 2005; Mayer & Rosenfeld, 2006; Thuresson et al., 2005). Thuresson and colleagues (2005), in a study that included only patients with diabetes, reported a similar prevalence of symptoms. Significant pain and discomfort in areas of the arm, neck, jaw, and back were also reported.
The fact that patients with diabetes experienced diminished physical functioning and higher levels of angina as measured by CCS class may explain why these patients were more than 2 times as likely to experience unusual fatigue during ACS. Fatigue is a symptom often observed in patients with ACS, and it has been reported by other investigators (DeVon et al., 2005; DeVon & Zerwic, 2003; Funk et al., 2001). Although fatigue can be a symptom of myocardial ischemia, it is also a common symptom of deconditioning, obesity, depression, and ventricular dysfunction—widespread problems for those with diabetes. In the current study, patients with diabetes had a higher body mass index, which could have contributed to fatigue. Long-standing fatigue resulting from diabetes may present an added risk for patients with diabetes because it may mask the symptoms associated with ACS.
The finding that patients with diabetes and those who are older have less chest pain during ACS is critical. There has been an assumption that patients with diabetes experience more silent myocardial ischemia, which has been demonstrated in the laboratory, but little evidence exists in the literature confirming that patients with diabetes have significantly less chest pain during ACS (Airaksinen, 2001; Canto et al., 2000; Culic, Eterovic, Miric, & Silic, 2002; Funk et al., 2001). Silent ischemia may be attributed to CAN, but CAN was not measured in these studies, so it remains to be validated in future research. It is likely that lack of chest pain may contribute to a decision to delay seeking treatment or to forgo care altogether if the health threat is labeled insignificant. Also, many patients with diabetes are already coping with symptoms associated with fluctuations in blood sugar. Therefore, symptoms of ACS may go undetected or may be mislabeled.
The hypothesis that symptoms of ACS would vary by duration of diabetes was supported; however, it was somewhat unexpected that there were no differences in symptoms between patients with diabetes of shorter duration (< 10 years) and those without diabetes. This finding may confirm that symptoms associated with microvascular changes and autonomic dysfunction are not manifested for more than 10 years.
Finally, the most significant predictors of the absence of chest pain were age and diabetes. Given that the incidence of CHD increases with age, the finding is important that a person with or without diabetes is 4% less likely to suffer chest pain as a person with the same diabetes status who is 1 year younger. Canto et al. (2000) reported that patients presenting with MI in the absence of chest pain are older than those presenting with chest pain (74.2 vs. 66.9 years). Brieger et al. (2004) also found that patients who present without chest pain are significantly older than those who present with chest pain.
Limitations to the study include a convenience sample and a lack of data on glycemic control before hospitalization. It is possible that the experience and severity of ACS symptoms can be partially dependent on blood sugar levels before or during an episode of ACS. Although the study was adequately powered to detect differences in symptoms between groups, a larger sample of patients with diabetes may have provided greater power to detect differences in symptoms with a smaller effect size.
There is evidence that patients with diabetes do experience more silent myocardial ischemia (Chico et al., 2005). The Detection of Ischemia in Asymptomatic Diabetes study found that more than 20% of patients with asymptomatic type 2 diabetes (no angina or anginal equivalents) had silent myocardial ischemia (Wackers et al., 2005). The cost of routine screening for CHD in patients with asymptomatic diabetes would be enormous and unnecessary because the presence of diabetes is a CHD equivalent (Miller, Redberg, & Wackers, 2006). It may be that older adults label chest pain differently; however, if they experience true silent ischemia, then they are at risk for delayed treatment, death, or other poor outcomes (Sheifer et al., 2000).
Conclusion
Nurses can use the findings of this study to educate patients with diabetes about the symptoms of ACS and to critically evaluate the significance of the clinical presentation of older patients and patients with diabetes. Patients with diabetes or with a documented history of ACS are at increased risk for cardiovascular complications and so benefit from ongoing education and support in managing their disease.
Acknowledgments
This study was supported by a grant from the National Institute for Nursing Research (No. R15-08870). We would like to thank Moshe Shapiro, biostatistician, for his assistance with statistical analyses and review of the manuscript, as well as Anne Bourguignon for her assistance with data collection.
References
- Airaksinen KE. Silent coronary artery disease—A feature of autonomic neuropathy or accelerated atherosclerosis? Diabetologia. 2001;44:250–266. doi: 10.1007/s001250051609. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Complications of diabetes in the United States. nd Retrieved November 15, 2007, from http://www.diabetes.org/diabetes-statistics.jsp.
- American Heart Association. Body mass index categories. nd Retrieved November 15, 2007 from http://www.americanheart.org/presenter.jhtml?identifier=4489#whatisbmiwhatis.
- Blendea MC, McFarlane SI, Isenovic ER, Gick G, Sowers JR. Heart disease in diabetic patients. Current Diabetes Reports. 2003;3:223–229. doi: 10.1007/s11892-003-0068-z. [DOI] [PubMed] [Google Scholar]
- Brieger D, Eagle KA, Goodman SG, Steg PG, Budaj A, White K, et al. Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high-risk group: Insights from the global registry of acute coronary events. Chest. 2004;126(2):461–469. doi: 10.1378/chest.126.2.461. [DOI] [PubMed] [Google Scholar]
- Bulpitt CJ, Palmer AJ, Battersby C, Fletcher AE. Association of symptoms of type 2 diabetic patients with severity of disease, obesity, and blood pressure. Diabetes Care. 1998;21(1):111–115. doi: 10.2337/diacare.21.1.111. [DOI] [PubMed] [Google Scholar]
- Campeau L. Letter: Grading of angina pectoris. Circulation. 1976;54(3):522–523. [PubMed] [Google Scholar]
- Canto JG, Shlipak MG, Rogers WJ, Malmgren JA, Frederick PD, Lambrew CT, et al. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA. 2000;283(4):3223–3229. doi: 10.1001/jama.283.24.3223. [DOI] [PubMed] [Google Scholar]
- Chico A, Tomas A, Novials A. Silent myocardial ischemia is associated with autonomic neuropathy and other cardiovascular risk factors in type 1 and type 2 diabetic subjects, especially in those with microalbuminuria. Endocrine. 2005;27(3):213–217. doi: 10.1385/ENDO:27:3:213. [DOI] [PubMed] [Google Scholar]
- Culic V, Eterovic D, Miric D, Silic N. Symptom presentation of acute myocardial infarction: Influence of sex, age, and risk factors. American Heart Journal. 2002;144(6):1012–1017. doi: 10.1067/mhj.2002.125625. [DOI] [PubMed] [Google Scholar]
- Dempsey SJ, Dracup K, Moser DK. Women’s decision to seek care for symptoms of acute myocardial infarction. Heart & Lung. 1995;24(6):444–456. doi: 10.1016/s0147-9563(95)80022-0. [DOI] [PubMed] [Google Scholar]
- DeVon HA, Penckofer SM, Zerwic JJ. Symptoms of unstable angina in patients with and without diabetes. Research in Nursing & Health. 2005;28(2):136–143. doi: 10.1002/nur.20067. [DOI] [PubMed] [Google Scholar]
- DeVon HA, Zerwic JJ. The symptoms of unstable angina: Do women and men differ? Nursing Research. 2003;52(2):108–118. doi: 10.1097/00006199-200303000-00007. [DOI] [PubMed] [Google Scholar]
- Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Funk M, Naum JB, Milner KA, Chyun D. Presentation and symptom predictors of coronary heart disease in patients with and without diabetes. American Journal of Emergency Medicine. 2001;19(6):482–487. doi: 10.1053/ajem.2001.27135. [DOI] [PubMed] [Google Scholar]
- Hollander JE. Acute coronary syndrome in the emergency department: Diagnosis, risk stratification, and management. In: Theroux P, editor. Acute coronary syndromes: A companion to Braunwald’s heart disease. 7. Philadelphia: Saunders; 2003. pp. 152–167. [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): Developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: Endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- Langer A, Freeman MR, Josse RG, Steiner G, Armstrong PW. Detection of silent myocardial ischemia in diabetes mellitus. American Journal of Cardiology. 1991;67(13):1073–1078. doi: 10.1016/0002-9149(91)90868-l. [DOI] [PubMed] [Google Scholar]
- Malecki D, Locke GR, Camilleri M, Zinsmeister AR, Yawn BP, Leibson C, et al. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Archives of Internal Medicine. 2000;160(8):2808–2816. doi: 10.1001/archinte.160.18.2808. [DOI] [PubMed] [Google Scholar]
- Manzella D, Paolisso G. Cardiac autonomic activity and type II diabetes mellitus. Clinical Science. 2005;108:93–99. doi: 10.1042/CS20040223. [DOI] [PubMed] [Google Scholar]
- Mayer DD, Rosenfeld A. Symptom interpretation in women with diabetes and myocardial infarction: A qualitative study. Diabetes Educator. 2006;32(6):918–924. doi: 10.1177/0145721706294262. [DOI] [PubMed] [Google Scholar]
- McSweeney JC, Crane PB. Challenging the rules: Women’s prodromal and acute symptoms of myocardial infarction. Research in Nursing & Health. 2000;23(2):135–146. doi: 10.1002/(sici)1098-240x(200004)23:2<135::aid-nur6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Miller TD, Redberg RF, Wackers FJT. Screening asymptomatic diabetic patients for coronary artery disease: Why not? Journal of the American College of Cardiology. 2006;48(4):761–764. doi: 10.1016/j.jacc.2006.04.076. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. New England Journal of Medicine. 2005;353(25):2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheifer SE, Canos MR, Weinfurt KP, Arora UK, Mendelsohn FO, Gersh BJ, et al. Sex differences in coronary artery size assessed by intravascular ultrasound. American Heart Journal. 2000;139(4):649–653. doi: 10.1016/s0002-8703(00)90043-7. [DOI] [PubMed] [Google Scholar]
- Thuresson M, Jarlov MB, Lindahl B, Svensson L, Zedigh C, Herlitz J. Symptoms and type of symptom onset in acute coronary syndrome in relation to ST elevation, sex, age, and a history of diabetes. American Heart Journal. 2005;150(2):234–242. doi: 10.1016/j.ahj.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Vinik AI, Freeman R, Erbas T. Diabetic autonomic neuropathy. Seminars in Neurology. 2003;23(4):365–372. doi: 10.1055/s-2004-817720. [DOI] [PubMed] [Google Scholar]
- Wackers FJ, Young LH, Inzucchi SE, Chyun DA, Davey JA, Barrett EJ, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects. Diabetes Care. 2005;27:1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- Zerwic JJ. Symptoms of acute myocardial infarction: Expectations of a community sample. Heart & Lung. 1998;27(2):75–81. doi: 10.1016/s0147-9563(98)90015-2. [DOI] [PubMed] [Google Scholar]