Abstract
In the clinical setting, antidotes are generally administered after the occurrence of a drug overdose. Therefore, the most pertinent evaluation of any new agent should model human exposure. This study tested whether acetaminophen (APAP) hepatotoxicity was reversed when S-adenosyl-L-methionine (SAMe) was administered after APAP exposure, similar to what occurs in clinical situations. Comparisons were made for potency between SAMe and N-acetylcysteine (NAC), the current treatment for APAP toxicity. Male C57BL/6 mice were fasted overnight and divided into groups: control (VEH), SAMe treated (SAMe), APAP treated (APAP), N-acetylcysteine treated (NAC), SAMe or NAC administered 1 h after APAP (SAMe+APAP) and (NAC+APAP), respectively. Mice were injected Intraperitoneal (ip) with water (VEH) or 250 mg/kg APAP (15 ml/kg). One 1h later, mice were injected (ip) with 1.25 mmol/kg SAMe (SAMe+APAP) or NAC (NAC+APAP). Hepatotoxicity was evaluated 4 h after APAP or VEH treatment. APAP induced centrilobular necrosis, increased liver weight and alanine transaminase (ALT) levels, depressed total hepatic glutathione (GSH), increased protein carbonyls and 4-hydroxynonenal (4-HNE) adducted proteins. Treatment with SAMe 1 hr after APAP overdose (SAMe+APAP) was hepatoprotective and was comparable to NAC+APAP. Treatment with SAMe or NAC 1 h after APAP was sufficient to return total hepatic glutathione (GSH) to levels comparable to the VEH group. Western blot showed reversal of APAP mediated effects in the SAMe+APAP and NAC+APAP groups. In summary, SAMe was protective when given 1 h after APAP and was comparable to NAC.
Keywords: Hepatotoxicity, Acetaminophen, Mice, S-adenosylmethionine, Antidote, N-acetylcysteine
1. INTRODUCTION
Acetaminophen (APAP, 4-hydroxyacetanilide) is a widely used over the counter agent possessing antipyretic and analgesic activity. The hospitalization rate due to accidental and intentional APAP overdose is estimated to be over 26,000 cases per year (Nourjah et al., 2006). Due to this high incidence rate, APAP is the most common cause of drug induced liver failure in the United States (Larson et al., 2005). APAP in excessive doses induces hepatic centrilobular necrosis (McJunkin et al., 1976).
APAP hepatic damage has been extensively examined in mice as hepatic centrilobular necrosis occurs within hours after administration (Mitchell et al., 1973; Jollow et al., 1973). APAP is bioactivated by cytochrome P450 to a highly reactive and toxic metabolite, N-acetyl-p-benzoquinoneimine (NAPQI) (Mitchell et al., 1973). Normally, NAPQI is detoxified by glutathione (GSH) conjugation. In overdose, hepatic GSH depletion occurs when NAPQI formation exceeds the available supply of GSH. Excess NAPQI not detoxified by GSH binds to cellular proteins (Nelson and Bruschi, 2003; Bulera et al., 1996), impairs mitochondrial respiration (Meyers et al.,1988; Burcham and Harman, 1991), opens the mitochondrial permeability transition pore (Masubuchi et al., 2005), increases oxidative stress (Bajt et al., 2004) and induces hepatic necrosis.
S-Adenosyl-L-methionine (SAMe) is an endogenous agent that is a critical precurosr for transmethylation and transsulfuration reactions. SAMe is a cofactor for many transmethylation reactions of amino acids, proteins, nucleotides and neurotransmitters. SAMe is also a vital precursor for the transsulfuration pathway which ultimately generates glutathione (Lu, 1998). Previous studies have shown that SAMe reduced APAP hepatotoxicity when administered just prior to APAP (Terneus et al., 2007; Valentovic et al., 2004; Bray et al., 1992; Carrasco et al., 2000; Song et al., 2004). SAMe administration just prior to APAP treatment decreased the severity of centrilobular necrosis in C57BL/6 male mice (Terneus et al., 2007). SAMe reduced the cytotoxicity of other hepatotoxins such as carbon tetrachloride and alcohol (Gasso et al., 1996; Song et al., 2003). SAMe pretreatment prevented carbon tetrachloride mediated hepatic toxicity and the development of liver fibrosis (Gasso et al., 1996). SAMe treatment of mice reduced alcohol mediated hepatic damage (Song et al., 2003). Partial reversal of alcohol induced cirrhotic liver has also been noted when SAMe was administered to humans (Lieber, 2002).
N-acetylcysteine (NAC) is the current clinical treatment for APAP overdose. It is well recognized that hepatic GSH depletion by generation of NAPQI is a crucial component in APAP hepatotoxicity resulting in elevated protein arylation by NAPQI (Nelson and Bruschi, 2003). NAC reduces APAP hepatic toxicity by increasing hepatic GSH levels as NAC provides cysteine as a precursor for GSH synthesis (Lauterburg et al., 1983; Corcoran et al., 1985 and 1986). In clinical situations, NAC is administered after the occurrence of an APAP overdose. NAC is most effective when administered to patients within 10-16 hr after APAP ingestion (Rumack et al., 1981).
The present study investigated whether SAMe would reduce APAP toxicity when administered after APAP exposure. The protective effect of SAMe would have the most relevance and potential clinical significance, if effective when administered after APAP exposure. This study compared the antidote effects of SAMe with an equimolar dose of NAC given 1 h after APAP treatment. These studies examined the potential beneficial effect of SAMe to reduce APAP hepatotoxicity relative to NAC, the current antidote.
2. MATERIALS AND METHODS
2.1 Chemicals
S-adenosyl-l-methionine (SAMe) was used in all studies as the toluenesulfonate salt (Sigma Chemical Co., St. Louis, Mo.). Glutathione, NADPH, 2-vinylpyridine and all other reagents were purchased from Sigma Chemical Company. The ALT reagent kit (TR-71021) was purchased from Thermo Electron Corporation (Louisville, CO). OxyBlot immunoblot kit was purchased from Upstate (Temecula, CA).
2.2 Animals
Male C57BL/6 mice (5 weeks old) weighing 16-20 grams were purchased from Hilltop Lab Animals Inc. (Scottsdale, PA). Mice were housed in a facility accredited by the American Association for Accreditation of Laboratory Animal Care. Mice were maintained under a controlled ambient temperature (21-23°C), humidity (40-55%) and 12 h light cycle (lights on 0600-1800 h). Mice were given a 7 day acclimation period prior to initiation of any procedures. Animals had free access to water and Purina Rodent Chow prior to initiation of any treatment. Mice were fasted overnight but had free access to water prior to APAP administration.
2.3 SAMe treatment 1 h after APAP
Mice were randomly divided into the following groups: vehicle treated (VEH); APAP treated (APAP), SAMe treated (SAMe) and SAMe treated plus APAP (SAMe+APAP). Each group contained 5-10 different mice/group. Mice were fasted for 16 h prior to any treatment. Mice were injected intraperitoneal (ip) at 0900 h, with 250 mg/kg APAP (15 ml/kg in water). Injections were administered at 0900 h in VEH and APAP animals in order to remove any confounding factors of circadian rhythm. The control animals (VEH group) were injected (ip) with water (15 ml/kg). One h after APAP or VEH treatment, mice were injected with 1.25 mmol/kg SAMe (500 mg/kg, 5 ml/kg in water). Toxicity was assessed 4 h after APAP injection as previous studies had shown toxicity was apparent at this time (Terneus et al., 2007). Beginning 4 h after APAP injection, mice were anesthetized with carbon dioxide and blood was collected by cardiac puncture. The livers were isolated, collected on ice, rinsed in ice cold Krebs buffer, blotted and weighed. Plasma was isolated from the blood and stored at 4°C until evaluation of ALT activity.
2.4 NAC treatment given 1 h after APAP
In a separate series of experiments mice (5-10/group) were randomly divided into the following groups: vehicle treated (VEH); APAP treated (APAP), NAC treated (NAC), NAC pretreated plus APAP (NAC+APAP). Mice were fasted as described above. Mice were injected, (ip), with 250 mg/kg APAP (15 ml/kg in water) or water (VEH) at 0900 h. One h after APAP or VEH injection (1000 h), mice were injected (ip) with 1.25 mmol/kg NAC (204 mg/kg, 5 ml/kg pH 7 in water). The NAC dose selected was equimolar to the SAMe treatment and has been used previously in our lab (Terneus et al., 2007). Plasma and liver was collected as described above 4 h after APAP administration.
2.5 Serum enzyme assay
Blood was collected for measurement of ALT levels. Plasma was collected and stored at 4°C until ALT levels were measured. ALT levels were measured using an enzymatic kit (TR-71021) obtained from Thermo Electron Corporation (Louisville, CO).
2.6 GSH Determination
Tissues (200 mg) were homogenized in 500 μl 0.5% sulfosalicylic acid and adjusted to a 1 ml volume. Total GSH was determined using glutathione reductase and NADPH coupled reaction with 5,5'-dithiobis(2-nitrobenzoic acid) as described previously (Valentovic et al., 2004); values were expressed as nmol/g tissue. Glutathione disulfide (GSSG) was measured following 2-vinylpyridine derivatization and expressed as nmol/g tissue.
2.7 Lipid peroxidation
Liver (200 mg) was homogenized in 1 ml Krebs buffer and the homogenizer probe was rinsed with an additional 1 ml of Krebs buffer. Lipid peroxidation was measured as described previously (Valentovic et al., 2004). The amount of malondialdehyde (MDA) was calculated based on a standard curve (range 1-40 nmol) using MDA (Aldrich, St. Louis, Mo) and expressed as umol MDA/mg protein.
2.8 Protein carbonyl (OxyBlot)
Protein carbonyls were assessed as previously described (Terneus et al., 2007). Hepatic tissue was homogenized in 5 volumes Krebs buffer pH 7.4 and the homogenizer probe was rinsed with 1 ml Krebs buffer. An aliquot was used for protein determination using a Coomasie Blue spectrophotometric method. The appearance of protein carbonyls was measured using a Protein Oxidation OxyBlot kit (Chemicon). The principle of the method is that modification of proteins by addition of carbonyl side chains due to oxidative stress are derivatized to 2,4-dinitirophenylhydrazone (DNP) following addition of 2,4-dinitrophenylhydrazine (DNPH). The antibody is specific for the DNP moiety on a protein.
Tissue samples were adjusted to ensure that equivalent volume and protein were added to gels. Samples and standards were run on polyacrylamide gel (12.5 % acrylamide). The gel was then transferred to a nitrocellulose membrane. To verify efficiency of transfer, the membrane was placed in Ponceau S stain for visualization. Ponceau S staining was used on all gels to ensure protein additions were comparable between all samples. The samples were run with DNP-derivatized molecular weight standards.
2.8 4-Hydroxynonenal (4-HNE) Adducts
Western blot was used to analyze for the presence of 4-HNE protein adducts as previously described (Harmon et al. 2006; Terneus et al., 2007). Samples and standards were run on polyacrylamide gel (12.5 % acrylamide) and transferred to a nitrocellulose membrane. To verify efficiency of transfer, the membrane was placed in Ponceau S stain for visualization. The membrane was then rinsed with H2O at room temperature under constant shaking to remove stain. Nonspecific protein binding was blocked with milk at room temperature under constant shaking for 1 hr. Rabbit polyclonal antibody to (E)-4-Hydroxynonenal (anti-HNE PAb) (1:1000; Alexis Biochemicals, ALX-210-767) was added and incubated overnight at 4°C under constant shaking. The membrane was then rinsed three times in tris-buffered saline tween (TBST). The secondary antibody, goat anti-rabbit linked with horseradish peroxidase (1:3000) in blocking buffer was added and incubated at room temperature under constant shaking for 90 min. The membrane was rinsed three times in TBST and developed using enhanced chemiluminescent substrate for visualization of 4HNE-adducted proteins.
2.9 Histology
A segment of liver was fixed in 15 ml neutral buffered formalin solution. The tissues were embedded in Paraplast and processed. The tissue was sectioned into approximately 4μm thickness and stained with hematoxylin and eosin (H&E). The tissues were viewed with a Nikon light microscope.
2.10 Statistical analysis
Values represent Mean ± SEM with n=5-10 animals/group. Differences between groups were analyzed using an analysis of variance (ANOVA) followed by a Tukey test (Sigma Stat, SPSS Inc. Chicago, IL). All statistical analyses were conducted using a 95% confidence interval with significance indicated by p values less than 0.05.
3. RESULTS
3.1 Protection by SAMe of APAP hepatic toxicity
SAMe treatment had no effect on body weight or hepatic function as liver to body weight ratios (Table 1) and ALT values (Table 2) were similar between the VEH and SAMe groups. APAP treatment induced hepatic toxicity as increased liver to body weight values (Table 1) and a 130 fold increase in ALT levels (Table 2) were apparent 4 h after injection when compared to the VEH and SAMe groups. ALT values in the SAMe+APAP group were only 3% of the APAP values. SAMe did not totally reverse hepatotoxicity as ALT values were higher in the SAMe+APAP group when compared to the VEH and SAMe groups (Table 2). However, the magnitude of ALT increase above control values was much higher in the APAP compared to the SAMe+APAP group.
Table 1.
Body and liver weight 4 h after APAP administration.
| Group | Treatment | Body weight (g) |
Liver weight (g/10g body wt.) |
|---|---|---|---|
| VEH | None | 16.4 ± 0.4 | 0.47 ± 0.01a |
| APAP | None | 16.8 ± 0.4 | 0.55 ± 0.01b |
| SAMe | SAMe | 17.0 ± 0.3 | 0.47 ± 0.01a |
| SAMe+APAP | SAMe | 16.4 ± 0.3 | 0.50 ± 0.01a |
| VEH | None | 18.0 ± 0.0 | 0.47 ± 0.01a |
| APAP | None | 17.6 ± 0.4 | 0.54 ± 0.01b |
| NAC | NAC | 17.6 ± 0.4 | 0.45 ± 0.01a |
| NAC+APAP | NAC | 18.0 ± 0.6 | 0.51 ± 0.01b |
Mice were treated with Vehicle (VEH, water ip injection 15 ml/kg), SAMe (1.25 mmol/kg, ip), n-acetylcysteine (NAC, 1.25 mmol/kg ip , 5 ml/kg), acetaminophen (APAP) administered as 250 mg/kg (ip, 15 ml/kg); SAMe administered 1 h after APAP (SAMe+APAP); or NAC administered 1 h after APAP (NAC+APAP). Statistical differences (p < 0.05) are denoted by superscripts within each treatment section. Values are reported as mean ± SEM with n = 4-10 animals/group. Groups with different superscripts denote statistical difference (p < 0.05) within each treatment experiment.
TABLE 2.
ALT Level 4 h post APAP, effect of SAMe recovery dose.
| Group | Treatment | ALT (U/L) |
|---|---|---|
| VEH | None | 96 ± 25a |
| APAP | None | 12,476 ± 407b |
| SAMe | SAMe | 84 ± 16a |
| SAMe+APAP | SAMe | 403 ± 107c |
| VEH | None | 74 ± 26a |
| APAP | None | 12,158 ± 1570b |
| NAC | NAC | 47 ± 10a |
| NAC+APAP | NAC | 801 ± 295c |
Mice were treated with Vehicle (VEH, water ip injection 15 ml/kg), SAMe (1.25 mmol/kg, ip), n-acetylcysteine (NAC, 1.25 mmol/kg, ip, 5 ml/kg), acetaminophen (APAP) administered as 250 mg/kg (ip, 15 ml/kg); SAMe administered 1 h after APAP (SAMe+APAP); or NAC administered 1 h after APAP (NAC+APAP). Statistical differences (p < 0.05) are denoted by superscripts within each treatment section. Values are reported as mean ± SEM with n = 4-10 animals/group. Groups with different superscripts denote statistical difference (p < 0.05) within each treatment experiment.
Liver morphology was normal in 5/5 of the VEH and SAMe treated mice (Figure 1, a and b) which was consistent with the normal ALT values (Table 2). Centrilobular necrosis was evident in all 5 APAP treated hepatic tissue 4 h after injection of 250 mg/kg APAP (Figure 1c). Extensive necrotic regions, loss of nuclei and cellular swelling were evident in the central vein region of all APAP treated mice. The extensive necrosis was consistent with the high ALT values (Table 2). Administration of SAMe 1 h after APAP greatly diminished hepatic damage (Figure 1d). The SAMe+APAP group showed marked improvement in hepatic integrity and almost normal morphology indicating that SAMe administered 1 h after APAP was able to reduce APAP mediated hepatic toxicity.
Figure 1. The effect of SAMe and NAC given 1 h after APAP treatment on hepatic tissue.
Hepatic tissue collected 4 h after ip injection of 250 mg/kg acetaminophen (APAP) from male C57BL/6 mice. Groups were vehicle (VEH), SAMe treated (SAMe), NAC treated, acetaminophen treated (APAP) and SAMe or NAC administered 1 h after APAP injection (SAMe+APAP) and (NAC+APAP), resp. SAMe and NAC were injected (ip) at a dose of 1.25 mmol/kg (5 ml/kg). Normal morphology was observed in: vehicle (VEH, panel A), SAMe (panel B) and NAC (Panel E) treated animals. Centrilobular necrosis was evident in the APAP (panel C) group. Treatment with SAMe (SAMe+APAP) or NAC (NAC+APAP) 1 h after APAP treatment was associated with less extensive centrilobular necrosis (panels D and F, resp.). All slides are H&E stained at 10 μm slice and 200x magnification.
3.2 NAC Protection of APAP
Further studies evaluated whether the protection afforded by SAMe for APAP hepatotoxicity was comparable to an equimolar dose of NAC. NAC did not impact hepatic function as liver to body weight ratios (Table 1) and ALT values (Table 2) were comparable to the VEH group. NAC administration 1 h after APAP tended to reduce liver to body weight values but the difference was not statistically significant when compared to the NAC group (Table 1). The increase in ALT values induced by APAP was less marked in the NAC+APAP as ALT values were lower in the NAC+APAP mice than in the APAP treated group.
Examination of H&E stained hepatic tissue verified normal morphology in 5/5 mice in the NAC group (Figure 1e). Treatment with NAC 1 h after APAP (NAC+APAP) reduced hepatic centrilobular necrosis (Figure 1f). However, focal necrosis was evident in the central vein region of the NAC+APAP treated mice indicating that the dose of NAC given in our study 1 h after APAP treatment did not totally prevent APAP mediated hepatic toxicity.
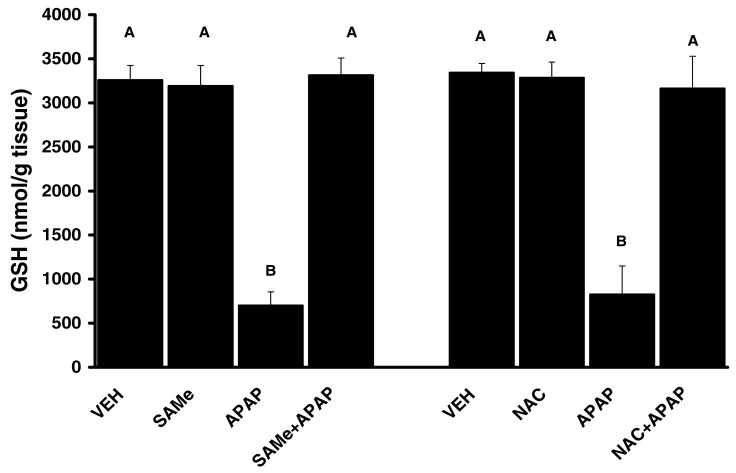
3.3 SAMe and NAC Prevent Hepatic GSH Depletion by APAP
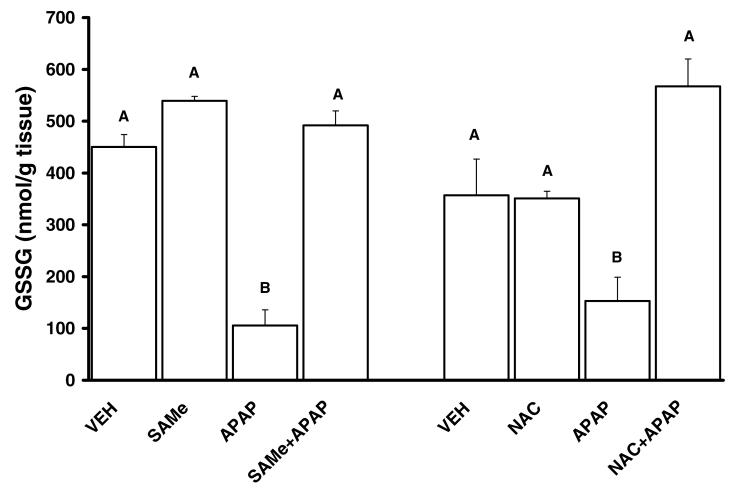
Total hepatic GSH levels were comparable between VEH, SAMe and NAC groups (Figures 2). APAP depleted total hepatic GSH levels by over 80% at 4 h when compared to VEH levels. SAMe administration 1 h after APAP restored total hepatic GSH to a level similar to VEH and SAMe levels (Figure 2). NAC treatment 1 h after APAP injection also returned total hepatic GSH levels comparable to VEH and NAC levels. APAP induced a decline in total glutathione disulfide (GSSG) levels when compared to VEH and SAMe treated groups (Figure 3). APAP also increased the percent of total GSH detected as GSSG suggesting an increase in oxidative stress (Table 3). Treatment with SAMe 1 h after APAP produced a higher level of GSSG when compared to the APAP group (Figure 3). NAC treatment 1 h after APAP resulted in a similar prevention of the decline in GSSG by APAP (Figure 3). The changes in GSSG suggested SAMe altered APAP mediated oxidative stress.
Figure 2. The Protective effect of SAMe and NAC on Total Hepatic Glutathione (GSH) Levels following APAP injection in C57BL/6 mice.
Animals were randomly divided into vehicle (VEH), acetaminophen treated (APAP), SAMe (500 mg/kg, 1.25 mmol/kg) treated (SAMe), N-acetylcysteine treated (NAC), SAMe administered 1 h after APAP injection (SAMe+APAP) and NAC administered 1 h after APAP injection (NAC+APAP). APAP was injected (ip) at a dose of 250 mg/kg (15 ml/kg). VEH treated were injected with water (15 ml/kg). Values represent Mean ± SEM, n=5 mice/group. Total hepatic glutathione levels were measured 4 h after injection (ip) of 250 mg/kg APAP. Unlike superscript letters indicate groups with statistical difference (p<0.05).
Figure 3. Glutathione disulfide (GSSG) Levels, rescue effect of SAMe and NAC administered 1 h after APAP.
Animals were randomly divided into vehicle (VEH), acetaminophen treated (APAP), SAMe (500 mg/kg, 1.25 mmol/kg) treated (SAMe), N-acetylcysteine treated (NAC), SAMe administered 1 h after APAP injection (SAMe+APAP) and NAC administered 1 h after APAP injection (NAC+APAP). APAP was injected (ip) at a dose of 250 mg/kg (15 ml/kg). VEH treated were injected with water (15 ml/kg). Glutathione disulfide (GSSG) levels were measured 4 h after APAP injection and 3 h after SAMe and NAC treatment. Unlike superscript letters indicate groups with statistical difference (p<0.05).
TABLE 3.
APAP increased percent of GSSG
| Group | Treatment | GSSG (% of total GSH) |
|---|---|---|
| VEH | None | 10.9 ± 2.0 |
| APAP | None | 17.9 ± 1.5 |
| SAMe | SAMe | 9.8 ± 1.1 |
| SAMe+APAP | SAMe | 19.8 ± 1.2* |
| VEH | None | 15.0 ± 1.0 |
| APAP | None | 24.7 ± 2.0 |
| NAC | NAC | 14.9 ± 1.1 |
| NAC+APAP | NAC | 19.1 ± 1.5 |
Mice were treated with Vehicle (VEH, water ip injection 15 ml/kg), SAMe (1.25 mmol/kg, ip), n-acetylcysteine (NAC, 1.25 mmol/kg ip, 5 ml/kg), acetaminophen (APAP) administered as 250 mg/kg (ip, 15 ml/kg), SAMe administered 1 h after APAP (SAMe+APAP); or NAC administered 1 h after APAP (NAC+APAP). Values are reported as mean ± SEM with n = 5 animals/group.
Statistical differences (p < 0.05) are denoted by an asterisk which indicates different (p<0.05) from SAMe group.
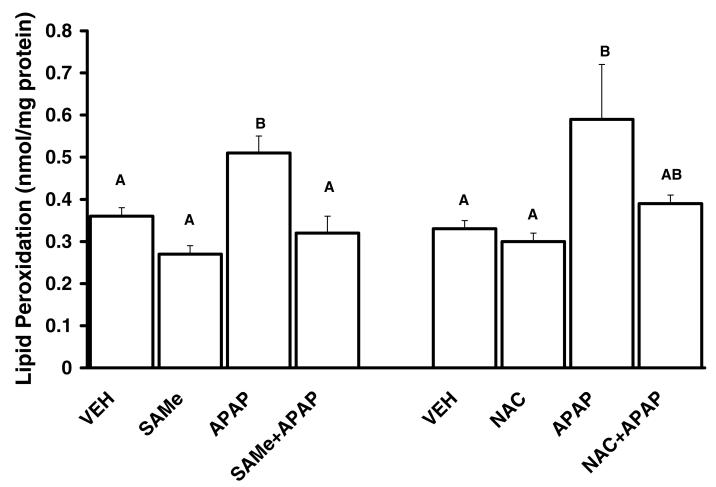
3.4 SAMe and NAC reduce APAP effect on lipid peroxidation
APAP increased lipid peroxidation when compared to the VEH, SAMe and NAC groups (Figure 4). Treatment with SAMe or NAC 1 h after APAP diminished lipid peroxidation to a level similar to SAMe, NAC and VEH levels (Figure 4). These results suggest that SAMe reduces the extent of APAP mediated oxidative stress and that the protection for lipid peroxidation was comparable between SAMe+APAP and NAC+APAP groups. Further, these results suggest that administration of SAMe 1 h after APAP was sufficient to reduce the extent of lipid peroxidation mediated by APAP.
Figure 4. Lipid peroxidation was reduced by SAMe and NAC treatment 1 h after APAP injection.
Animals were randomly divided into vehicle (VEH), acetaminophen treated (APAP), SAMe (500 mg/kg, 1.25 mmol/kg) treated (SAMe), N-acetylcysteine treated (NAC), SAMe administered 1 h after APAP injection (SAMe+APAP) and NAC administered 1 h after APAP injection (NAC+APAP). APAP was injected (ip) at a dose of 250 mg/kg (15 ml/kg). VEH treated were injected with water (15 ml/kg). Lipid peroxidation was reported as nmol malondialdehyde (MDA) per mg protein. Lipid peroxidation was measured in hepatic tissue collected 4 h after APAP injection. Values represent MEAN ± SE. Groups with dissimilar superscripts are different (p<0.05) from each other. SAMe and NAC administered 1 h after APAP diminished the extent of hepatic lipid peroxidation.
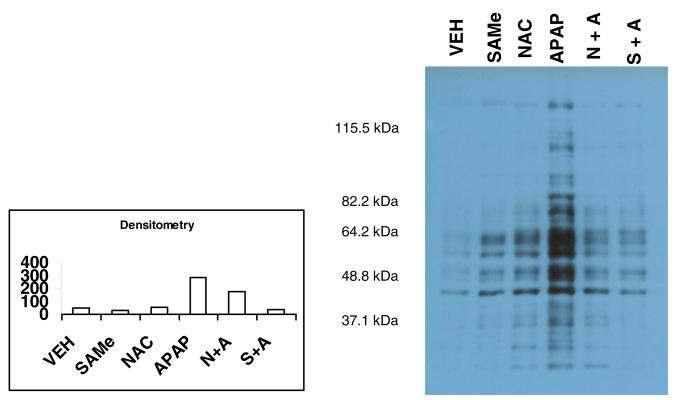
3.5 APAP Effects on Hepatic 4-HNE Adducted Proteins and Protein Carbonyls
Western blot analysis of hepatic tissue indicated that protein carbonyls were increased within 4 h by APAP treatment (Figure 5). The OxyBlot showed comparable band intensity for hepatic tissue from VEH, SAMe and NAC groups suggesting minimal oxidative stress. Treatment 1 h after APAP injection with SAMe greatly diminished the positive band intensity detected by OxyBlot. SAMe and NAC administered 1 h after APAP reduced the appearance of band intensity at the 46 and 64 kDa region (Figure 5). These results suggest that SAMe treatment 1 h after APAP injection was sufficient to reduce the oxidative damage mediated by APAP when measured 4 h after APAP injection. A similar decrease in the appearance of positive band intensity was observed in the NAC+APAP group suggesting that treatment 1 h after APAP injection with SAMe or NAC was sufficient to reduce the oxidative stress mediated by APAP.
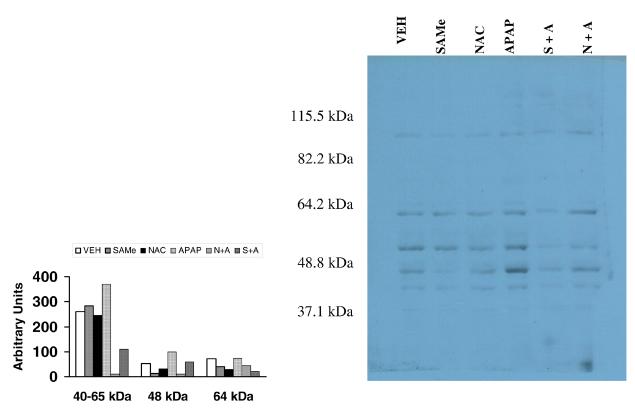
Figure 5. Protein carbonyls in hepatic tissue 4 h after APAP treatment and 3 h after SAMe or NAC treatment.
Hepatic tissue was isolated 4 h after APAP or VEH injection. SAMe and NAC were administered 1 h after APAP injection (ip). The lanes within the gel represent VEH (lane 1), 1.25 mmol/kg SAMe (lane 2), 1.25 mmol/kg NAC (lane 3), 250 mg/kg APAP (lane 4), combination of NAC and APAP (NAC+APAP lane 5) and a combination of SAMe and APAP (lane 6) as described in the methods. Western blots were performed using an OxyBlot Kit to detect protein carbonyls. Each lane in the gel was loaded with the same volume and concentration of protein. Densitometry was performed and expressed in arbitrary units.
Western blot of hepatic tissue showed minimal 4-hydroxynonenal (4-HNE) adducted proteins in VEH, SAMe and NAC treated groups (Figure 6). Immunopositive 4-HNE adducted proteins were detected at 45, 50 and 62 kDa in the APAP treated group to a greater extent than the VEH, SAMe and NAC groups. These results indicate that lipid aldehyde byproducts of lipid peroxidation were alkylating hepatic proteins to a greater extent following APAP treatment. Administration of SAMe 1 h after APAP diminished the magnitude of immunopositive stained proteins. A similar protective effect was noted when NAC was administered 1 h after APAP treatment. These results provide further support that SAMe administered as an antidote 1 h after APAP administration was protective as it reduced the severity of 4-HNE adducted hepatic proteins.
Figure 6. 4-HNE adducted hepatic proteins 4 h after APAP treatment and 3 h after SAMe or NAC treatment.
Hepatic tissues were collected from mice 4 h after injection (ip) with VEH (lane 1), 1.25 mmol/kg SAME (lane 2), 1.25 mmol/kg NAC (lane 3), 250 mg/kg APAP (lane 4), 1.25 mmol/kg SAMe administered 1 h after 250 mg/kg APAP (SAMe+APAP, lane 5) and 1.25 mmol/kg NAC administered 1 h after 250 mg/kg APAP (NAC+APAP, lane 6) as described in the methods. Western blots were performed using an anti-4HNE primary antibody with a peroxidase conjugated secondary antibody. Gels were loaded with the same volume and concentration of protein based on mass. Bands were detected via chemiluminescence. Densitometry was performed and expressed in arbitrary units.
4. DISCUSSION
NAC is the drug of choice for APAP overdose. Unfortunately, NAC administration may induce a variety of side effects. Adverse effects for NAC include anaphylactic reaction, nausea, vomiting and diarrhea (Holdiness, 1991; Bonfiglio et al., 1992; Kao et al., 2003; Kerr et al., 2005). The latter GI side effect would ultimately diminish hepatic delivery of NAC. Consequently, other treatment modalities with fewer side effects would be beneficial. The major side effects associated with clinical use of SAMe at a dose of 1600 mg/day was a 20% incidence of headache and diarrhea but none of the individuals had to stop SAMe usage (Rosenbaum et al., 1990).
Reports by other laboratories have shown that certain agents were beneficial at reducing APAP hepatotoxicity. Several agents such as clofibrate (Manautou et al., 1996), ribose cysteine (Lucas et al., 2000); L-cysteine glutathione mixed disulfides (Berkeley et al., 2003), antioxidants (Oz et al., 2004), 2(RS)-n-propylthiazolidine-4Rcarboxylic acid (Srinivsan et al., 2003) and NAC (Lauterburg et al., 1983) reduced APAP hepatic toxicity. However, all these compounds were successful in reducing APAP hepatic toxicity when administered prior to APAP exposure. Very few agents have been reported to reduce APAP hepatotoxicity when administered after APAP exposure similar to what is needed for an antidote. NAC, the therapeutic antidote for APAP overdose, is one of the few agents to reduce toxicity when administered after APAP exposure to mice (Salminen et al., 1998; James et al., 2003). The effectiveness of NAC was inversely dependent on the time NAC was administered after APAP. NAC was most effective in reducing APAP toxicity in mice when administered within 1 h of APAP treatment and was least beneficial if administered 3 or 4 h after APAP overdose, respectively (Salminen et al 1998; James et al., 2003). Salminen and associates (1998) also reported that covalent binding by NAPQI was not reduced if NAC was administered 1 h after APAP treatment but NAC still successfully reduced APAP hepatic toxicity. These findings would suggest that covalent binding by NAPQI is a component of APAP toxicity but additional events also contribute to hepatic damage. Furthermore, since NAC given 1 h after APAP overdose still provides protection without reducing APAP mediated hepatic arylation (Salminen et al., 1998; James et al., 2003) the mechanism for protection involves processes independent of covalent binding when given after APAP exposure. It is also possible that the protection mediated by SAMe when administered after APAP overdose is due to processes independent of covalent binding. Further studies will be necessary to determine whether this hypothesis is true.
In the clinical setting, NAC is administered every 4 h for as many as 17 doses over the course of 72 h following APAP overdose. Since APAP has a relatively short half-life of 2 to 4 h in humans, NAC treatment for multiple days must at least partially assist with cellular damage and increased oxidative stress. The mechanism for NAC in APAP recovery would be to mediate protection by providing cysteine for increased glutathione synthesis (Lauterburg et al., 1983) as NAC administered after APAP injection does not prevent formation of APAP protein adducts (James et al., 2003).
The current study showed that SAMe was effective in reducing APAP toxicity when it was administered after APAP exposure. These results show that SAMe was similar to NAC in reducing APAP hepatotoxicity when administered 1 h after APAP treatment at equivalent mmol doses. Time course studies in mice treated with NAC at varying times after APAP overdose reported maximum protection occurred when NAC was administered within 1 h after APAP (James et al., 2003). Consequently, our studies were designed to administer SAMe and NAC 1 h after APAP.
SAMe is normally present in the body and acts as a major methyl donor for transmethylation reactions. Transmethylation reactions are essential in maintaining normal cell function, especially in the liver, for transmethylation of phospholipids, proteins, nucleotides and neurotransmitters (Chang et al., 1996; Lieber and Packer, 2002). SAMe is also a substrate for the transsulfuration pathway which converts SAMe to homocysteine and ultimately to glutathione (Lu, 1998). The mechanism for SAMe protection of APAP toxicity may involve both the transsulfuration and transmethylation pathways. The transsulfuration pathway would help assist in recovery of depleted hepatic GSH which would be similar to the mechanism for NAC. SAMe would also stimulate the transmethylation pathways which would assist in recovery by generating new intermediates within surviving hepatocytes. SAMe is a critical cofactor in transmethylation reactions of membrane phospholipids such as phosphatidyethanolamine to phosphatidylcholine (Stramentinoli et al., 1979). Transmethylation reactions would be anticipated to increase membrane fluidity and reduce membrane damage.
SAMe provided protection of APAP mediated alterations in hepatic proteins due to oxidative stress. APAP induced an increase in oxidative stress as bands positive for protein carbonyls (Figure 5) and 4-HNE (Figure 6) were observed in hepatic tissue. 4-HNE positively stained bands between 40- 64 kDa were apparent with 4 h following APAP treatment (Figure 6). 4-HNE, an aldehyde generated during lipid peroxidation, can impair cellular function. 4-HNE attaches to cellular proteins by a 1,4-Michael addition to specific amino acids including cysteine and lysine (Esterbauer et al., 1991). The reduced intensity for the 4-HNE adducted proteins in the SAMe+APAP would indicate that SAMe reduced oxidative stress in hepatic tissue even when administered 1 h after APAP treatment.
It is unlikely that SAMe attenuation of APAP hepatic toxicity was due to inhibition of cytochrome P450 conversion of APAP to NAPQI. APAP has an approximate half-life of 1 h in mice (Fischer et al., 1981) which would provide sufficient time to generate the toxic metabolite prior to SAMe administration. Based on the pharmacokinetics of APAP in mice, approximately 50% of APAP is already eliminated when SAMe was administered which would suggest that the mechanism for SAMe protection does not involve alteration of biotransformation.
In summary, the hepatic toxicity of APAP was reduced by equimolar doses (1.25 mmol/kg) of SAMe or NAC 1 h after administration of a toxic dose of APAP was effective in reducing hepatic damage. This study was also the first to provide a comparison of APAP hepatic toxicity when SAMe and NAC were administered 1 h after APAP treatment. These results suggest that SAMe, at least in our model, has some potential as an antidote for APAP toxicity.
5. ACKNOWLEGEMENT
This work was supported in part by NIH Grant 5P20RR016477 to the West Virginia IDeA Network for Biomedical Research Excellence.
Footnotes
This work was a component of the doctoral dissertation by M. Terneus.
NOTICE: None of the authors have a conflict of interest with this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- Bajt ML, Knight TR, LeMaster JL, Jaeschke H. Acetaminophen induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl Cysteine. Toxicol. Sci. 2004;80:343–349. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- Berkeley LI, Cohen JF, Crankshaw DL, Shirota FN, Nagasawa HT. Hepatoprotection by L-cysteine-glutathione mixed disulfide, a sulfhydryl-modified prodrug of glutathione. J Biochem Mol Toxicol. 2003;17:95–97. doi: 10.1002/jbt.10069. [DOI] [PubMed] [Google Scholar]
- Bonfiglio MF, Traeger SM, Hulisz DT, Martin BR. Anaphylactoid reaction to intravenous acetylcysteine associated with electrocardiographic abnormalities. Ann Pharmacother. 1992;26:22–25. doi: 10.1177/106002809202600105. [DOI] [PubMed] [Google Scholar]
- Bray GP, Tredger JM, Williams R. S-Adenosylmethionine protects against APAP hepatotoxicity in two mouse models. Hepatology. 1992;15:297–301. doi: 10.1002/hep.1840150220. [DOI] [PubMed] [Google Scholar]
- Bulera SJ, Cohen SD, Khairallah EA. Acetaminophen-arylated proteins are detected in hepatic subcellular fractions and numerous extra hepatic tissue in CD-1 and C57Bl/6 mice. Toxicology. 1996;109:85–99. doi: 10.1016/0300-483x(96)03309-4. [DOI] [PubMed] [Google Scholar]
- Burcham PC, Harman AW. Acetaminophen Toxicity in Site-Specific Mitochondrial Damage in Isolated Mouse Hepatocytes. J. Biol. Chem. 1991;255:5049–5054. [PubMed] [Google Scholar]
- Carrasco R, Perez-Mateo M, Gutierrez A, Esteban A, Mayol MJ, Caturla J, Ortiz P. Effect of different doses of S-adenosyl-L-methionine on paracetamol hepatotoxicity in a mouse model. Methods Find Exp Clin Pharmacol. 2000;22(10):737–40. doi: 10.1358/mf.2000.22.10.802290. [DOI] [PubMed] [Google Scholar]
- Chang PK, Gordon RK, Tai J, Zeng GC, Doctor BP, Pardhasaradhi K, McGann PP. S-Adenosylmethionine and methylation. FASEB J. 1996;10:471–480. [PubMed] [Google Scholar]
- Corcoran GB, Todd EL, Racz WJ, Hughes H, Smith CV, Mitchell JR. Effects of N-acetylcyteine on the disposition and metabolism of APAP in mice. J. Pharmacol. Exp. Ther. 1985;232:857–863. [PubMed] [Google Scholar]
- Corcoran GB, Wong BK. Role of glutathione in prevention of acetaminophen induced hepatotoxicity by N-acetyl-L-cysteine in vivo: Studies with Nacetyl-D-cysteine in mice. J. Pharmacol. Exp. Ther. 1986;238:54–61. [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Rad. Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Fischer LJ, Green MD, Hardman AW. Levels of acetaminophen and is metabolites in mouse tissue after a toxic dose. J. Pharmacol Expt Ther. 1981;219:281–286. [PubMed] [Google Scholar]
- Gasso M, Rubio M, Varela G, Cabre M, Caballeria J, Alonso E, Deulofem R, Camps J, Gimenez A, Pajares M, Pares A, Mato JM, Rodes J. Effects of S-adenosylmethionine on lipid peroxidation and liver firbrogenesis in carbon tetrachloride induced cirrhosis. J. Hepatology. 1996;25:200–205. doi: 10.1016/s0168-8278(96)80074-2. [DOI] [PubMed] [Google Scholar]
- Harmon RC, Kiningham KK, Valentovic MA. Pyruvate reduces 4-aminophenol in vitro toxicity. Toxicol Appl. Pharmacol. 2006;213:179–186. doi: 10.1016/j.taap.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991;20:123–134. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
- James LP, McCullough SS, Lamps LW, Hinson JA. Effect of nacetylcysteine on acetaminophen toxicity in mice: relationship to reactive nitrogen and cytokine formation. Tox. Sci. 2003;75:458–467. doi: 10.1093/toxsci/kfg181. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther. 1973;187:195–202. [PubMed] [Google Scholar]
- Kao LW, Kirk MA, Furbee RB, Mehta NH, Skinner JR, Brizendine EJ. What is the rate of adverse events after oral N-acetylcysteine administered by the intravenous route to patients with suspected acetaminophen poisoning? Ann Emerg Med. 2003;42:741–750. doi: 10.1016/s0196-0644(03)00508-0. [DOI] [PubMed] [Google Scholar]
- Kerr F, Dawson A, Whyte IM, Buckley N, Murray L, Graudins A, Chan B, Trudinger B. The Australasian Clinical Toxicology Investigators Collaboration randomized trial of different loading infusion rates of N-acetylcysteine. Ann Emerg Med. 2005;45:402–408. doi: 10.1016/j.annemergmed.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiodt FV, Ostapowicz G, Shaki AO, Lee WM. Acetaminophen sets record in the United States: Number 1 analgesic and number 1 cause of acute liver failure. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of action of Nacetylcysteine in the protection against the hepatotoxicity of APAP in rats in vivo. J Clin Invest. 1983;71:980–991. doi: 10.1172/JCI110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS. S-Adenosyl-L-methionine: its role in the treatment of liver disorders. Am J Clin Nutr. 2002;76(suppl 1):1183S–1187S. doi: 10.1093/ajcn/76/5.1183S. [DOI] [PubMed] [Google Scholar]
- Lieber CS, Packer L. S-Adenosylmethionine: molecular, biological and clinical aspects- an introduction. Am J Clin Nutr. 2002;76(5):1148S–1150S. doi: 10.1093/ajcn/76/5.1148S. [DOI] [PubMed] [Google Scholar]
- Lu SC. Regulation of hepatic glutathione synthesis. Sem. Liver Dis. 1998;18:331–334. doi: 10.1055/s-2007-1007168. [DOI] [PubMed] [Google Scholar]
- Lucas AM, Henning G, Dominick PK, Whiteley HE, Roberts JC, Cohen SD. Ribose cysteine protects against acetaminophen induced hepatic and renal toxicity. Toxicologic Path. 2000;28:697–704. doi: 10.1177/019262330002800510. [DOI] [PubMed] [Google Scholar]
- Manautou JE, Tveit A, Hoivik DJ, Khairallah EA, Cohen SD. Protection by clofibrate against acetaminophen hepatotoxicity in male CD-1 mice is associated with an early increase in biliary concentration of acetaminophen-glutathione adducts. Toxicol Appl Pharmacoogy l. 1996;140:30–38. doi: 10.1006/taap.1996.0194. [DOI] [PubMed] [Google Scholar]
- Masubuchi Y, Sudua C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen induced liver injury in mice. J of Hepatology. 2005;42:110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- McJunkin B, Barwick KW, Little WC, Winfield JB. Fatal massive hepatic necrosis following acetaminophen overdose. JAMA. 1976;236:1874–1875. [PubMed] [Google Scholar]
- Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen induced inhibition of hepatic mitochondrial respiration in mice. Toxicol. Appl. Pharmacol. 1988;93:378–387. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- Nelson SD, Bruschi SA. Mechanisms of Acetaminophen induced liver disease. In: Kaplowitz N, DeLeve LD, editors. Drug Induced Liver Disease. Marcel Dekker; NY: 2003. pp. 287–325. [Google Scholar]
- Nourjah P, Ahmad SR, Karwoski C, Willy M. Estimates of acetaminophen (paracetamol)-associated overdoses in the United States. Pharmacoepidemiology and Drug Safety. 2006;15:398–405. doi: 10.1002/pds.1191. [DOI] [PubMed] [Google Scholar]
- Oz HS, McClain CJ, Nagasawa HT, Ray MB, de Villiers WJS, Chen T. Diverse antioxidants protect against acetaminophen hepatotoxicity. J. Biochem Mol. Toxicol. 2004;18:361–368. doi: 10.1002/jbt.20042. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JF, Fava M, Falk WE, Pollack MH, Cohen LS, Cohen BM, Zubenko GS. The antidepressant potential of oral S-adenosyl-l-methionine. Acta Psychiatr Scand. 1990;81(5):432–436. doi: 10.1111/j.1600-0447.1990.tb05476.x. [DOI] [PubMed] [Google Scholar]
- Rumack BH, Peterson RG, Koch GG, Amara IA. Acetaminophen overdose, 662 cases with evaluation of oral acetylcysteine treatment. Arch Intern Med. 1981;141(3 Spec):380–385. doi: 10.1001/archinte.141.3.380. [DOI] [PubMed] [Google Scholar]
- Salminen WF, Voellmy R, Roberts SM. Effect of N-acetylcysteine on heat shock protein induction by acetaminophen in mouse liver. J. Pharmacol Exp Ther. 1998;286:519–524. [PubMed] [Google Scholar]
- Song Z, McClain CJ, Chen T. S-Adenoyslmethionine protects against acute alcohol induced hepatotoxicity in mice. J Nutr. Biochem. 2003;14:591–597. doi: 10.1016/s0955-2863(03)00116-5. [DOI] [PubMed] [Google Scholar]
- Song Z, Zhou Z, Chen T, Hill D, Kang J, Barve S, McClain C. S-Adenosylmethionine (SAMe) protects against acute alcohol induced hepatotoxicity in mice. J. Nutr. Biochem. 2003;14:591–597. doi: 10.1016/s0955-2863(03)00116-5. [DOI] [PubMed] [Google Scholar]
- Song Z, McClain CJ, Chen T. S-Adenosylmethionine protects against acetaminophen induced hepatotoxicity in mice. Pharmacology. 2004;71(4):99–208. doi: 10.1159/000078086. [DOI] [PubMed] [Google Scholar]
- Srinivasan C, Williams WM, Nagasawa HT, Chen TS. Effects of 2(RS)-n-propylthiazolidine-4(R)-carboxylic acid on extrahepatic sulfhydryl levels in mice treated with acetaminophen. Biochem Pharmacol. 2001;61:925–931. doi: 10.1016/s0006-2952(01)00539-1. [DOI] [PubMed] [Google Scholar]
- Stramentinoli G, Gualano M, Galli-Kienle M. Intestinal absorption of Sadenosyl-L-methionine. J Pharmacol Exp Ther. 1979;209(3):323–6. [PubMed] [Google Scholar]
- Terneus MV, Kiningham KK, Carpenter AB, Sullivan SB, Valentovic MA. Comparison of S-Adenosyl-L-methionine and N-acetylcysteine protective effects on acetaminophen hepatic toxicity. J Pharmacol Exp Ther. 2007;320(1):99–107. doi: 10.1124/jpet.106.111872. [DOI] [PubMed] [Google Scholar]
- Valentovic MA, Terneus MV, Harmon RC, Carpenter AB. S-Adenosylmethionine (SAMe) attenuates acetaminophen hepatotoxicity in C57BL/6 mice. Toxicol. Letters. 2004;154:165–174. doi: 10.1016/j.toxlet.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Wang X, Cederbaum AI. S-Adenosyl-L-methionine attenuates hepatotoxicity induced by Agonist Jo2 Fas antibody following Cyp2E1 induction in mice. J Pharmacol Expt Ther. 2006;317:44–52. doi: 10.1124/jpet.105.098004. [DOI] [PubMed] [Google Scholar]