Abstract
Objective
Reduced cardiac parasympathetic activity, as indicated by a reduced level of clinic or ambulatory high-frequency heart rate variability (HF-HRV), is associated with an increased risk for atherosclerosis and coronary artery disease. We tested whether the reduction in HF-HRV to a psychological stressor relative to a baseline level is also associated with subclinical coronary or aortic atherosclerosis, as assessed by calcification in these vascular regions.
Method
Spectral estimates of 0.15 to 0.40 Hz HF-HRV were obtained from 94 postmenopausal women (61–69 years) who engaged in a 3-minute speech-preparation stressor after a 6-minute resting baseline. A median of 282 days later, electron beam tomography (EBT) was used to measure the extent of coronary and aortic calcification.
Results
In univariate analyses, a greater reduction in HF-HRV from baseline to speech preparation was associated with having more extensive calcification in the coronary arteries (rho = −0.29, p = .03) and in the aorta (rho = −0.22, p = .06). In multivariate analyses that controlled for age, education level, smoking status, hormone therapy use, fasting glucose, high-density lipoproteins, baseline HF-HRV, and the stressor-induced change in respiration rate, a greater stressor-induced reduction in HF-HRV was associated with more calcification in the coronary arteries (B = −1.21, p < .05), and it was marginally associated with more calcification in the aorta (B = −0.92, p = .09).
Conclusion
In postmenopausal women, a greater reduction in cardiac parasympathetic activity to a psychological stressor from baseline may be an independent correlate of subclinical atherosclerosis, particularly in the coronary arteries.
Keywords: aorta, atherosclerosis, coronary, calcification, electron beam tomography, heart rate variability, parasympathetic, stress
INTRODUCTION
Psychological stressors suppress cardiac parasympathetic nervous system activity in most individuals. This stressor-induced suppression of cardiac parasympathetic activity is documented in a growing number of studies that use high-frequency heart rate variability (HF-HRV) as an indirect measure of parasympathetic (vagal) control over time-related variations in heart rate that occur within the average range of adult respiratory frequencies (≅0.15–0.40 Hz; (1–5)). Individual differences in the magnitude of the change in HF-HRV elicited by psychological stressors, however, have been noted (e.g., (4,5)). Furthermore, it has been speculated that individuals who show large-magnitude reductions in cardiac parasympathetic activity to psychological stressors may be at an increased risk for atherosclerosis and for other end points that are associated with coronary artery disease (CAD (6–8)). This speculation is supported indirectly by epidemiological studies that indicate that lower levels of clinic and ambulatory HRV, which may reflect reduced cardiac parasympathetic activity, are associated with an increased risk of angina pectoris, congestive heart failure, coronary stenosis, myocardial infarction, terminal ventricular arrhythmias, and cardiac death (9–19). This speculation also complements the longstanding cardiovascular reactivity hypothesis, which posits that individuals who show large-magnitude or exaggerated cardiovascular (e.g., blood pressure) responses to stressors are at an increased for negative cardiovascular health outcomes, including atherosclerosis (20).
Although an increasing number of cross-sectional and prospective studies have demonstrated that exaggerated cardiovascular reactions to psychological stressors are associated with subclinical markers of atherosclerosis (e.g., increased carotid intima-media thickness; see (21) for review), no studies to our knowledge have tested whether stressor-induced reductions in HF-HRV or other estimates of cardiac parasympathetic reactivity are similarly associated with subclinical markers of atherosclerosis.
Accordingly, we tested whether individuals who show a large-magnitude reduction in HF-HRV, indicating a reduction in cardiac parasympathetic activity, to a psychological stressor also have increased levels of subclinical coronary or aortic atherosclerosis. To test this hypothesis, we measured HF-HRV at rest and in response to a speech preparation stressor in a sample of postmenopausal women from the Healthy Women Study (HWS (22)). A median of 282 days after HF-HRV measurement, we obtained electron beam tomography (EBT) estimates of coronary and aortic calcification, noninvasive subclinical markers of atherosclerosis that predict both cardiac morbidity and mortality (23–26). We also measured and accounted for other factors that have been associated with calcification and HF-HRV: age, smoking status, the use of hormone therapy, body mass index (BMI), resting blood pressure, and fasting concentrations of lipids and glucose.
METHOD
Participants
Our report is based on data from 94 61- to 69-year-old postmenopausal women who were enrolled in the HWS (22). The HWS is a longitudinal investigation (initiated in 1983–1985) of biobehavioral risk factors for cardiovascular disease among women as they transition through menopause. All HWS participants were contacted initially by mailings to women who had driver’s licenses in Allegheny County, Pennsylvania. Of the 2405 women interviewed about eligibility criteria, 541 consented to participate. Women were eligible for the entry into HWS if they were aged 42 to 50 years, had menstruated in the prior 3 months, did not have menopause by surgery, had a DBP below 100 mm Hg, and were not on medications that could affect targeted cardiovascular risk factors (e.g., estrogen, antihypertensive, cholesterol-lowering, glucoregulatory, thyroid, or psychotropic medications). After enrollment, HWS participants were given a clinical examination, after which they reported their menstrual status monthly. Women were then classified as postmenopausal when they reported that their menstruation had ceased and/or when they had used hormone replace therapy (HRT) for 12 months. Women again completed clinical evaluations at the time of postmenopausal classification and every several years thereafter. The EBT scanning protocol was added to the HWS study in 1997, and it was made available to women who were at least 5 years postmenopause. In 2000, all women who were still active participants in the HWS and who still lived within traveling distance of the University of Pittsburgh were invited to participate in the psychological stress testing protocol that is described below. Of the HWS who met these criteria, 232 consented to participate in the psychological stress protocol, and data were available for the present report from 129 women who completed the psychological stress protocol on the day of or before having an EBT scan.
Of these 129 women, we excluded from our analyses the data from 35 women who met 1 of the following criteria: 1) a prior diagnosis of CAD (n = 1) or the reported use of medication for CAD (n = 3); 2) current treatment for cancer (n = 9) or type I or II diabetes (n = 6); 3) a fasting glucose concentration that exceeded 148 mg/dL, a value that was 3 standard deviations (SDs) above the mean glucose concentration (96.7 mg/dL) of the present sample (n = 2); and 4) the reported use of alpha- or beta-blockers (n = 14).
As shown in Table 1, the results reported here were derived from a relatively healthy sample of 94 older (61–69 years of age) postmenopausal women (89 white, 4 black, and 1 Asian/Pacific Islander) with few current smokers, an average BMI, and normal levels of resting systolic blood pressure (SBP) and diastolic blood pressure (DBP) and plasma concentrations of lipids and glucose.
TABLE 1.
Sample Characteristics and Univariate Spearman Rank Correlations With Coronary and Aortic Calcium Scores
| Coronary Calcium | Aortic Calcium | ||||
|---|---|---|---|---|---|
| Characteristic | Value | ||||
| r | p | r | p | ||
| Age (yrs) | 64.5 (1.6) | 0.10 | — | 0.18 | 0.09 |
| Education Level (Ns) | −0.17 | 0.11 | −0.20 | 0.06 | |
| High School or less | 13 | ||||
| Some college | 28 | ||||
| 4 year degree | 21 | ||||
| Graduate degree | 32 | ||||
| Smoking status (N smokers) | 8 | 0.25 | 0.02 | 0.32 | 0.001 |
| HT use (N current HT users) | 44 | −0.14 | 0.19 | −0.16 | 0.13 |
| BMI (kg/m²) | 28.2 (5.7) | 0.06 | — | 0.10 | — |
| Waist circumference (inches) | 33.6 (5.6) | 0.10 | — | 0.19 | 0.07 |
| Physical activity (kcal/week) | 1721.3 (1692.7) | −0.07 | — | −0.08 | — |
| Total cholesterol | 208.7 (39.4) | −0.09 | — | 0.05 | — |
| HDL (mg/dl) | 58.2 (15.8) | −0.05 | — | −0.18 | 0.08 |
| LDL (mg/dl) | 124.1 (34.3) | −0.12 | — | 0.08 | — |
| Median triglycerides (mg/dl) | 105.0 (64.3) | −0.02 | — | 0.05 | — |
| Glucose (mg/dl) | 96.7 (11.8) | 0.18 | 0.08 | 0.30 | 0.004 |
| Baseline SBP (mm Hg) | 124.9 (18.4) | 0.11 | — | 0.12 | — |
| Baseline DBP (mm Hg) | 72.3 (8.7) | 0.05 | — | −0.11 | — |
| Baseline HR (bpm) | 67.9 (11.3) | 0.06 | — | −0.03 | — |
| Baseline respiration rate (breaths/min) | 13.8 (2.9) | 0.12 | — | −0.02 | — |
| Baseline HF-HRV (ln units) | 4.52 (1.15) | 0.06 | — | −0.03 | — |
| Δ From baseline to speech preparation | |||||
| Δ SBP (mm Hg) | 18.0 (13.3) | −0.01 | — | 0.02 | — |
| Δ DBP (mm Hg) | 7.5 (7.2) | −0.01 | — | 0.03 | — |
| Δ HR (bpm) | 6.8 (6.5) | 0.004 | — | −0.11 | — |
| Δ Respiration rate (breaths/min) | 1.0 (1.3) | 0.14 | 0.23 | 0.13 | 0.25 |
| Δ HF-HRV (ln units) | −0.29 (0.76) | −0.26 | 0.03 | −0.22 | 0.06 |
HT = hormone therapy; BMI = body mass index; HDL = high-density lipoproteins; LDL = low-density lipoproteins; SBP = systolic blood pressure; DBP = diastolic blood pressure; bpm = beats per minute; HF-HRV = high-frequency heart rate variability; Δ = Speech preparation task - baseline change score.
Note. Values are shown as means with standard deviations in parentheses, unless indicated otherwise. Triglyceride values were natural log transformed before statistical analyses.
Dash indicates a p value >.25.
All women gave their informed consent before, and were not remunerated for, their participation. The University of Pittsburgh’s Institutional Review Board approved all protocols for the HWS and the present study.
Psychological Stress Protocol
Before the psychological stress protocol, women refrained from caffeine, tobacco products, and exercise for 3 hours, and from drinking alcohol for 12 hours. On arrival at the laboratory, the participant 1) provided her informed consent, 2) provided demographic information, 3) reported her current medication use, 4) described her medical and surgical history, 5) had her height and weight measured, 6) had electrocardiographic (ECG) electrodes applied to her, and 7) had a blood pressure cuff placed over her brachial artery for automated blood pressure measurements. The participant was then seated in a comfortable chair in a climate-controlled testing room.
Once seated, the participant rested quietly for a 10-minute baseline period and then completed 3 laboratory stressors: 1) a mirror-image tracing task (3 minutes), in which she traced—as quickly and accurately as possible—the outline of an image while viewing the figure in its mirrored appearance; 2) a public-speaking task, in which she prepared (3 minutes) and delivered (3 minutes) a speech about a stressful situation (see subsequently); and 3) a cold-pressor test (approximately 1 minute), in which she sat quietly while a bag of iced water was held on her forehead. The cold pressor test was always presented last, and the mirror-image and speech tasks were presented in a random counterbalanced order. The participant was given a 10-minute rest period between tasks, and she was continuously supervised through a closed circuit monitor.
ECG signals for heart rate and HF-HRV analyses were recorded only during the last 6 minutes of the initial 10-minute baseline period (allowing for 4 minutes of adaptation) and only during the entire 3-minute speech preparation task. ECG signals for HF-HRV derivation were not recorded during the speech delivery, mirror-image tracing, and cold pressor tasks to avoid the possible confounding effects of vocalization and movement-related activity on derived estimates of HF-HRV (1,27). During the baseline and speech preparation periods, blood pressure measurements were taken approximately 90 seconds apart using an automated blood pressure device (IBS SD-700A; IBS Corp., Waltham, MA). All blood pressure measurements that were obtained during the baseline period were averaged, as were the 2 speech preparation task measurements. During the same baseline and speech preparation task periods, minute-by-minute averages of respiration rate (in breaths per minute) were obtained by a nasal cannula that was connected to an Ohmeda 4700 OxiCap Monitor (Ohmeda, Louisville, CO). Thus, we report only on estimates of HF-HRV and other cardiorespiratory variables that were derived from the initial resting baseline period and from the speech preparation task. Cardiovascular measures from the remaining tasks will be presented in a separate report.
Details of the speech preparation task are as follows: the participant was asked to imagine that a police officer issued her a $100 ticket for failing to stop at a stop sign. The task of the participant was to prepare a speech about what she would say to a judge in explanation of this traffic violation. To help prepare the speech, the participant was given an index card that instructed her to discuss 1) the events that led to the traffic violation, 2) whether the ticket was issued wrongfully, and 3) the city’s responsibility for posting plain-view traffic signs. The participant was asked to give a well-developed speech, and she was told that the speech would be videotaped and evaluated for content and style. The woman was left alone for the 3-minute preparation task. After the preparation task, an experimenter entered the room, took away the index card, and instructed the woman to give her speech while facing a wall-mounted video camera.
Electrocardiographic Monitoring and High-Frequency Heart Rate Variability Assessment
During the baseline and speech preparation task, ECG signals were obtained from 3 silver–silver chloride electrodes (Conmed; Andover Medical, Haverhill, MA) that were positioned in a modified lead II configuration. The ECG signal was digitized (12 bit), sampled (at 1000 Hz), and stored for offline processing using LabView acquisition software (National Instruments Corp., Austin, TX).
Before calculating HF-HRV, R-wave markers in the ECG signal were evaluated for artifacts by visual inspection and by an automatic artifact detection algorithm (28) that was implemented in a customized software package (Mindware Heart Rate Variability Scoring Module, version 2.16; Mindware Technologies Ltd., Columbus, OH). Suspected artifacts were corrected manually (<1% of all R-waves in the present sample were corrected). After artifact correction, minute-by-minute estimates of heart rate and HF-HRV were determined in accordance with current guidelines (1,29). First, for each minute of the baseline and the speech preparation task, a 60-second time series of interbeat intervals (IBIs; the time in milliseconds between sequential ECG R spikes) was created from an interpolation algorithm that had a 250-ms sample time. This 60-second IBI time series was then 1) linearly detrended, 2) mean-centered, and 3) tapered using a Hamming window. Spectral-power values were then determined (in ms²/Hz) with fast Fourier transformations, and the power values in the 0.15- to 0.40-Hz spectral bandwidth were integrated (ms²). Before statistical analyses, a natural log (ln) transformation was applied to the spectral power values to correct for distributional violations, and this value was taken as an indicator of HF-HRV.
We averaged the minute-by-minute estimates of heart rate, HF-HRV, and respiration rate for the baseline period and for the 3-minute speech preparation task. For each woman, the average baseline value of HF-HRV, heart rate, respiration rate, SBP, and DBP was subtracted from its corresponding speech preparation value to yield a single task-minus-baseline change score. In addition to baseline values, these change scores were used as independent variables in regression models that predicted coronary and aortic calcification.
Electron Beam Tomography Assessment of Coronary and Aortic Calcification
A median of 282 days (range, 0–754 days) after psychological stress testing, an EBT-trained technician used an Imatron C150 scanner (Imatron, South San Francisco, CA) and its densitometric program to assess the extent of calcification in the coronary arteries and in the aorta. During coronary scanning, 3-mm thick contiguous transverse images were obtained from the level of the aortic root to the apex of the heart. During aortic scanning, 6-mm thick contiguous images were obtained from the aortic arch to the iliac bifurcation. Each contiguous image (100-ms exposure) was acquired during the same phase of the cardiac cycle using electrocardiographic triggering. From these images, we derived Agatston (30) calcium scores for the coronary arteries and for the aorta. A previous report from the HWS (31) demonstrated a high EBT scan-to-scan reproducibility of coronary and aortic calcium scores (interclass correlations = 0.99 for coronary calcium and 0.98 for aortic calcium). In the present sample, coronary and aortic calcium scores showed a moderate univariate Spearman rank correlation (rho = 0.39, p < .001).
Consistent with prior reports from the HWS (31–33), coronary and aortic calcium scores were positively skewed and could not be corrected with data transformations. The median coronary calcium score was 7.9 (range, 0–878), with the lower 25% of the women having a score of 0 and the upper 75% having a score of 77.7 or more. The median aortic calcium score was 156.5 (range, 0–7938), with the lower 25% of the women having a score of 15.0 or less and the upper 75% having a score of 860.5 or more. Available guidelines (34) indicate that coronary calcium scores ≤10 reflect little or no identifiable atherosclerotic plaque burden; scores>10 are taken to indicate minimal to extensive atherosclerotic plaque burden. To be consistent with these guidelines, we used a dichotomization procedure to classify women as having a low (coronary calcium score ≤10 = 0; n = 48 in the present sample) or a high (calcium score > 10 = 1; n = 46) coronary calcium score. As yet, there are no clinical guidelines for aortic calcification. To be consistent with our coronary scoring, which formed 2 groups with approximately equal sample sizes, we used a comparable dichotomization (median split) approach to classify women has having a low aortic (calcium score ≤156.5 = 0) or a high (calcium score > 156.5 = 1) aortic calcium score. These low and high calcium scores were used as criterion (dependent) variables in binary logistic regression models.
Assessment of Calcification Risk Factors
We assessed factors that have been associated with coronary and aortic calcification and with HF-HRV in prior reports (e.g., 31–33). Specific factors assessed at the time of psychological stress testing included age, current smoking status (coded as 0 = nonsmoker and 1 = current smoker), baseline SBP and DBP, BMI, current use of hormone therapy (HT; coded as 0 = nonHT user and 1 = current HT user), education level (coded as 0 = a high school degree or less; 1 = an associate degree, vocational training, or some college education; 2 = a 4-year college degree; and 3 = a graduate degree), and kilocalories per week spent in leisure physical activity (assessed by the Paffenbarger Activity Questionnaire (35)). Factors assessed at the time of the woman’s clinical evaluation that coincided with her EBT scan included 12-hour fasting concentrations of total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglycerides, and glucose (see (36) for details regarding serum assay analyses). Because of their skewed distribution, triglyceride values were corrected with a natural log transformation before statistical analyses.
Data Analysis
Covariate Selection
Using Spearman rank correlations, we first evaluated univariate relationships between nondichotomized coronary and aortic calcium scores and calcification risk factors. Using the results from these correlation analyses (summarized in Table 1), age and those factors that correlated either with coronary calcium or with aortic calcium at p < .10 were subsequently included as covariates in logistic regression analyses that predicted the high versus low coronary and aortic calcium criterion variables that were described previously. Using these selection criteria, we included as covariates age, smoking status, HDL, and fasting glucose in all models (see Table 1). No other factor assessed at the time of psychological stress testing or at the time of the EBT scan showed a univariate relationship with coronary or with aortic calcification that was statistically significant (p < .05) or that met our covariate selection criteria (p < .10).
Prediction of Coronary and Aortic Calcification
After covariate selection, we used binary logistic regression analyses to predict the dichotomized criterion variables of coronary and aortic calcification (in which 0 = low and 1 = high). These criterion variables were predicted by the primary explanatory (independent) variables of baseline HF-HRV and the HF-HRV task - baseline change score, in addition to the covariates described previously. We evaluated the unstandardized regression coefficient (B) for each independent variable and covariate in the model along with each variable’s odds ratio (OR) and 95% OR confidence interval (OR CI).
Missing Data
Of the 94 women included the present sample, 15 had missing HF-HRV data because of ECG data acquisition failure (n = 11) or because the participant declined to complete the speech preparation task (n = 4). Compared with women with complete data, t tests and chi-squared analyses showed that women with missing HF-HRV data did not differ in age, coronary or aortic calcium scores, or in any other factor shown in Table 1 (all p’s > .35). Thus, the final sample size for the univariate correlations and the logistic regression analyses on HF-HRV is based on 79 of the 94 women tested. All other univariate correlations (shown in Table 1) are from the full sample of 94 participants.
RESULTS
Effects of the Speech Preparation Task on High-Frequency Heart Rate Variability, Respiration Rate, Heart Rate, and Blood Pressure
HF-HRV decreased from the baseline period (mean, 4.55; SD, 1.15 ln units) to the speech preparation task (mean, 4.26; SD, 1.22 ln units), indicating an overall reduction in cardiac parasympathetic activity during this psychological stressor (t = 3.40, p < .001) by a paired samples t test. In conjunction with this reduction in HF-HRV, respiration rate increased from baseline (mean, 13.68; SD, 3.02 breaths per minute) to the speech preparation task (mean, 14.70; SD, 3.50 breaths per minute), t = 4.21, p < .001 by a paired samples t test. In addition, a greater reduction from baseline to speech preparation in HF-HRV correlated with a greater increase in respiration rate across women in the present sample (r = −0.26, p < .05). This correlation suggested that an increase in respiratory rate to the speech preparation stressor may have contributed to the stressor-induced reduction in HF-HRV (1). Therefore, the change in respiration rate from baseline to the speech preparation task included an additional covariate in the logistic regression models that predicted coronary and aortic calcification from the stressor-induced change in HF-HRV.
In addition to the overall reduction in HF-HRV and increase in respiratory rate, average heart rate, SBP, and DBP increased from the baseline period (HR: mean, 68.56, SD, 9.11 beats per minute [bpm]; SBP: mean, 120.30, SD, 17.90 mm Hg; DBP: mean, 72.09, SD, 8.33 mm Hg) to the speech preparation task (HR: mean, 75.30, SD, 11.37 bpm; SBP: mean, 138.31, SD, 23.84 mm Hg; DBP: mean, 79.59, SD, 9.47 mm Hg, all t’s >8.39, p’s < .001 by paired samples t tests. Collectively, these findings indicated that the speech preparation task increased cardiovascular reactivity, which parallels results from prior studies that have used a speech preparation task as a psychological stressor (e.g., 2–5).
Associations Between High-Frequency Heart Rate Variability and Coronary and Aortic Calcification
Women who had coronary calcium scores greater than or equal to 10 showed a significantly greater unadjusted mean decrease of in HF-HRV from baseline to speech preparation than women with coronary calcium scores less than 10 (mean Δ, −0.57, SD, 0.79 ln units versus mean Δ = −0.01, SD, 0.62 ln units, respectively), t = 3.37, p < .001 by an independent samples t test. Furthermore, women with aortic calcium scores greater than 156 showed a significantly greater unadjusted mean decrease in HF-HRV from baseline to speech preparation compared with women with aortic calcium scores less than or equal to 156 (mean Δ, −0.42, SD, 0.71 ln units versus mean Δ, −0.07, SD, 0.72 ln units), t = 2.10, p < .05 by an independent samples t test. For illustration, Figure 1 shows the unadjusted change in HF-HRV from baseline to the speech preparation task in women with scores falling into each coronary and aortic calcification tertile.
Figure 1.
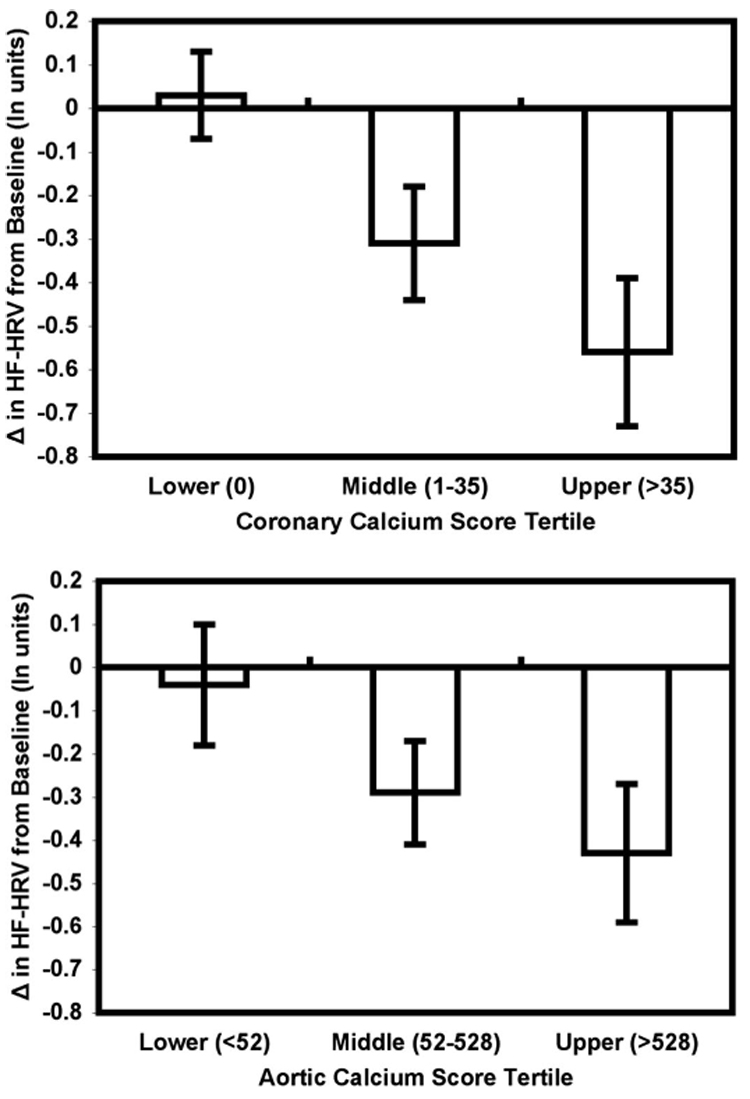
Unadjusted mean change (± standard error of mean) in high-frequency heart rate variability (HF-HRV) from a resting baseline to a speech preparation task in 79 postmenopausal women with Agatston calcium scores falling in the lower, middle, and upper tertiles of the coronary (bottom) and aortic (top) calcium score distributions. The range of calcium scores for each tertile is shown in parentheses along the abscissa. Changes in HF-HRV to the speech preparation task were assessed a median of 282 days (range, 0–754 days) before coronary and aortic calcium scores were obtained.
After controlling for age, smoking status, use of HT, fasting HDL and glucose concentrations, baseline HF-HRV, and the stressor-induced change in respiration rate, a greater reduction in HF-HRV from baseline to the speech preparation task predicted having coronary calcium scores greater than 10 (see Table 2). A similar analysis for aortic calcification showed that a greater reduction in HF-HRV was marginally related to having aortic calcium scores greater than 156 (see Table 3).
TABLE 2.
Binary Logistic Regression Analysis Predicting Coronary Calcium
| Variable | B | p | OR | 95% OR CI |
|---|---|---|---|---|
| Age | 0.14 | 0.42 | 1.15 | 0.82 to 1.63 |
| Education Level | 0.004 | 0.98 | 1.004 | 0.61 to 1.67 |
| Smoking Status | 2.74 | 0.04 | 1.07 | 1.01 to 1.85 |
| HDL | 0.01 | 0.54 | 1.01 | 0.98 to 1.05 |
| Glucose | 0.04 | 0.17 | 1.04 | 0.98 to 1.09 |
| Baseline HF-HRV | 0.20 | 0.49 | 1.22 | 0.70 to 2.12 |
| Δ Respiration Rate | 0.02 | 0.88 | 1.02 | 0.76 to 1.34 |
| Δ HF-HRV | −1.21 | 0.01 | 0.30 | 0.11 to 0.78 |
OR = odds ratio; CI = confidence interval; HDL = high-density lipoproteins; HF-HRV = high-frequency heart rate variability; Δ = speech preparation task - baseline change score.
Note. Coronary calcium, the criterion variable, was coded as low (calcium score ≤10 = 0) vs. high (calcium score >10 = 1). Aortic calcium, the criterion variable, was coded as low (calcium score ≤156 = 0) vs. high (calcium score >156 = 1). Education level was coded as 0 = a high school degree or less; 1 = some college; 2 = a 4-yr college degree; and 3 = a graduate degree); smoking status was coded as nonsmoker = 0 and current smoker = 1.
TABLE 3.
Binary Logistic Regression Analysis Predicting Aortic Calcium
| Variable | B | p | OR | 95% OR CI |
|---|---|---|---|---|
| Age | 0.63 | 0.01 | 1.88 | 1.15 to 3.06 |
| Education Level | −0.15 | 0.62 | 0.86 | 0.48 to 1.55 |
| Smoking Status | 2.19 | 0.15 | 1.12 | 0.94 to 2.12 |
| HDL | −0.05 | 0.03 | 0.95 | 0.91 to 0.99 |
| Glucose | 0.10 | 0.004 | 1.10 | 1.03 to 1.19 |
| Baseline HF-HRV | −0.28 | 0.44 | 0.75 | 0.37 to 1.54 |
| Δ Respiration Rate | 0.10 | 0.50 | 1.11 | 0.82 to 1.50 |
| Δ HF-HRV | −0.92 | 0.09 | 0.40 | 0.14 to 1.15 |
HDL = high-density lipoproteins; HF-HRV = high-frequency heart rate variability; Δ = speech preparation task - baseline change score.
Note. Aortic calcium, the criterion variable, was coded as low (calcium score ≤156 = 0) versus high (calcium score >156 = 1). Education level was coded as 0 = a high school degree or less; 1 = some college; 2 = a 4 year college degree; and 3 = a graduate degree); smoking status was coded as nonsmoker = 0 and current smoker = 1.
In a supplementary set of logistic regression analyses, we found that the change in heart rate, SBP, and DBP from baseline to speech preparation did not predict high versus low coronary or aortic calcification when 1) we considered these change scores separately or together in the same models, or when 2) we included or excluded the same covariates that were used in the HF-HRV regression models (all p’s > .63). Overall, these null results parallel the lack of univariate associations between calcification and these cardiovascular reactivity variables, which are summarized in Table 1.
Finally, apart from its association with the stressor-induced change in respiration rate (described previously) and in heart rate (r = −0.26, p = .02), the change in HF-HRV from baseline to the speech preparation task was not associated with any other factor assessed at the time of psychological stress testing or at the time of EBT scanning (all p’s > .20). Furthermore, the current use of HT was not associated with baseline HF-HRV or with the stressor-induced change in HF-HRV. Specifically, women who reported that they currently used HT at the time of psychological stress testing showed a baseline HF-HRV (mean, 4.47; SD, 1.16 ln units) and an average decrease in HF-HRV from the baseline to the speech preparation task (mean Δ = −0.18; SD, 0.78 ln units) that were comparable to the baseline HF-HRV (mean, 4.55; SD, 1.16 ln units) and decrease in HF-HRV (mean Δ = −0.36; SD = 0.74 ln units) that were shown by women who did not report using HT (all t’s <1, p’s > .33) by independent samples t tests.
DISCUSSION
The main finding of the present study was that a greater reduction in HF-HRV from a resting baseline to a period of psychological stress (elicited by preparing an impromptu speech) was associated with more extensive calcification in the coronary arteries and in the aorta. This finding was obtained in a relatively healthy sample of postmenopausal women without known CAD. In addition, after statistical control for age, tobacco use, HDL, fasting glucose, baseline HF-HRV, and the stressor-induced change in respiration rate, a greater stressor-induced reduction in HF-HRV continued to show an association with more extensive calcification in the coronary arteries, but showed only a marginal association with calcification in the aorta. Thus, a greater reduction in cardiac parasympathetic activity during psychological stress, as reflected by a greater reduction in HF-HRV, may be a potential risk factor for subclinical atherosclerosis, particularly in the coronary arteries.
Prior studies indicate that exaggerated blood pressure reactions to psychological stressors are associated with a greater risk for subclinical carotid atherosclerosis, which is taken in support of the cardiovascular reactivity hypothesis of cardiovascular disease risk (20,21). Blood pressure reactions to psychological stressors, however, are crude indicators of autonomic activation. As such, they reveal little about how specific patterns of sympathetic or parasympathetic cardiac reactivity to psychological stressors may be associated with atherosclerosis risk or with other CAD-related end points. Moreover, in the present study, stressor-induced changes in heart rate and blood pressure were not associated with coronary or with aortic calcification. In contrast, a more specific indicator of cardiac parasympathetic reactivity to the speech preparation stressor, the stressor-induced change in HF-HRV, was associated with subclinical calcification. These findings may thus indicate that more specific indicators of autonomic activation to stress are more closely associated with subclinical atherosclerosis, particularly in the coronary arteries, than are more nonspecific autonomic cardiovascular measures, such as heart rate and blood pressure reactivity. This speculation, however, should be weighed in balance with 3 primary limitations of the present study.
First, the present study tested only postmenopausal women, which may limit generalizations from this sample. There is evidence that pre- and postmenopausal women differ in their cardiac autonomic reactivity to stress, and there is mixed evidence that the use of hormone therapy after menopause may also influence cardiac autonomic reactivity (37–40). As has been found in previous studies, we did not observe an effect of hormone therapy use on HF-HRV at rest or in response to our psychological stressor. However, it is still possible that the relationship between HF-HRV (at rest or in response to psychological stress) and calcification differs between women before and after menopause. Furthermore, there is also evidence that men and women differ in their autonomic and cardiovascular responses to psychological stressors (40). Men and women also differ in their rates of cardiovascular disease (and calcification) development, and these differential disease rates may interact with age-related changes in hormonal status (41). It is possible, therefore, that decreased HF-HRV during psychological stress may differentially relate to atherosclerotic markers such as calcification between men and women. Second, the present study tested relatively older (61–69 years) women. It is well established that calcification increases and cardiac parasympathetic activity, as assessed by HF-HRV, decreases with age (1,29,42,43). In the present study, however, we did not observe a statistically significant association between age and HF-HRV, which may have been the result of the restricted age range or the excellent health of our older sample. Future work is thus needed to test whether the present results generalize to a younger sample or to a sample with a larger variation in age and health status. Third, although we assessed coronary and aortic calcification after we assessed HF-HRV at the psychological stress testing session, both of these assessments were conducted within a median time period of less than 1 year. Thus, the present findings could be considered cross-sectional. Consequently, the present findings do not exclude the possibility that latent pathology, reflecting structural or functional changes in vascular tissue that are associated with the development of calcification, affected the ability of the parasympathetic nervous system to control high-frequency heart rate variations.
Furthermore, the absence of a relationship between baseline (resting) HF-HRV and calcification in the present study contrasts with the results of a prior cross-sectional study by Colhoun et al. (44) in which reduced levels of resting HRV were associated with the presence of coronary calcification (calcium scores >0) in a mixed sample of type I diabetic and nondiabetic individuals. Differences between the present results and those of that study may be the result of at least 2 factors. First, whereas we used HF-HRV as a continuous predictor of high versus low coronary and aortic calcification, Calhoun et al. used a different measure of HRV, total HRV, as a trichotomized and cross-sectional correlate of the presence versus the absence of coronary calcification. Second, Colhoun et al. reported that their measure of total HRV did not correlate with the presence of calcification when diabetic and nondiabetic individuals were analyzed separately, or when age, SBP, and BMI were used as covariates. Thus, the differences between our results and those of Calhoun et al. may be the result of differences in study design, HRV, and calcification quantification, or in the evaluation of individuals for whom different covariates may be more or less relevant.
Finally, the mechanisms by which a greater reduction in HF-HRV to psychological stress may be associated with an increased risk for atherosclerosis are unclear. One speculation (8) is that a pattern of increased sympathetic and decreased parasympathetic cardiac activity during psychological stress may increase atherosclerotic risk by promoting endothelial dysfunction, which is a precursor to the development of atherosclerosis. In support of this speculation is accumulating evidence from studies on nonhuman animals, which suggest that increased sympathetic activation during periods of stress may contribute to endothelial dysfunction by promoting blood flow disruptions (shear stress) that injure the endothelium or by facilitating inflammatory processes that form atherosclerotic plaques: intimal macrophage invasion, smooth muscle cell proliferation, lipid accumulation, and plaque calcification (45–49). There is also evidence that efferent cholinergic activity of the vagus nerve, the parasympathetic arm of the autonomic nervous system, may inhibit the cellular activation of macrophages and inflammatory cytokines—processes that are presumptively involved in atherosclerotic plaque formation (50). However, it remains to be determined in prospective studies whether greater reductions in cardiac parasympathetic activity through the vagus (as estimated by greater reductions in HF-HRV) during psychological stress predict a greater risk for endothelial dysfunction or atherosclerosis.
To summarize, the present results indicate that a greater reduction in cardiac parasympathetic activity, as indexed by a greater reduction in HF-HRV, to a psychological stressor is associated with more extensive subclinical atherosclerosis, as indexed by coronary calcification. These results complement prior evidence indicating that reduced levels of clinic and ambulatory HF-HRV are associated with an increased risk for atherosclerosis and other CAD-related end points (9–19). Further work is needed to determine whether the present results generalize to premenopausal women, to men, or to younger individuals. Further work is also needed to clarify the potential mechanisms by which cardiac parasympathetic reactions to psychological stress are associated with atherosclerosis.
Acknowledgments
This research was supported by NIH grants HL65111, HL65112, HL28266, and by K01-MH-070616. We thank Leslie A. Mitrik and Heather L. Koston for their technical assistance. We also thank Karen S. Quigley and Natasha Tokowicz for their comments on an earlier draft of the manuscript.
Glossary
- ANOVA
analysis of variance
- BMI
body mass index
- BPM
beats per minute
- DBP
diastolic blood pressure
- EBT
electron beam tomography
- HDL
high-density lipoproteins
- HF-HRV
high-frequency heart rate variability
- HT
hormone therapy
- IBI
interbeat interval
- LDL
low-density lipoproteins
- ln
natural log
- SBP
systolic blood pressure
REFERENCES
- 1.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 2.Hawkley LC, Burleson MH, Poehlmann KM, Berntson GG, Malarkey WB, Cacioppo JT. Cardiovascular and endocrine reactivity in older females: intertask consistency. Psychophysiology. 2001;38:863–872. doi: 10.1111/1469-8986.3860863. [DOI] [PubMed] [Google Scholar]
- 3.Burleson MH, Malarkey WB, Cacioppo JT, Poehlmann KM, Kiecolt-Glaser JK, Berntson GG, Glaser R. Postmenopausal hormone replacement: effects on autonomic, neuroendocrine, and immune reactivity to brief psychological stressors. Psychosom Med. 1998;60:17–25. doi: 10.1097/00006842-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Gianaros PJ, Quigley KS, Mordkoff JT, Stern RM. Gastric myoelectrical and autonomic cardiac reactivity to laboratory stressors. Psychophysiology. 2001;38:642–652. [PMC free article] [PubMed] [Google Scholar]
- 5.Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: the psychometrics of respiratory sinus arrhythmia and preejection period. Psychophysiology. 1994;31:412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- 6.Sloan RP, Shapiro PA, Bagiella E, Myers MM, Gorman JM. Cardiac autonomic control buffers blood pressure variability responses to challenge: a psychophysiologic model of coronary artery disease. Psychosom Med. 1999;61:58–68. doi: 10.1097/00006842-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz AR, Gerin W, Davidson KW, Pickering TG, Brosschot JF, Thayer JF, Christenfeld N, Linden W. Toward a causal model of cardiovascular responses to stress and the development of cardiovascular disease. Psychosom Med. 2003;65:22–35. doi: 10.1097/01.psy.0000046075.79922.61. [DOI] [PubMed] [Google Scholar]
- 8.Harris KF, Matthews KA. Interactions between autonomic nervous system activity and endothelial function: a model for the development of cardiovascular disease. Psychosom Med. 2004;66:153–164. doi: 10.1097/01.psy.0000116719.95524.e2. [DOI] [PubMed] [Google Scholar]
- 9.Hayano J, Yamada A, Mukai S, Sakakibara Y, Yamada M, Ohte N, Hashimoto T, Fujinami T, Takata K. Severity of coronary atherosclerosis correlates with the respiratory component of heart rate variability. Am Heart J. 1991;121:1070–1079. doi: 10.1016/0002-8703(91)90664-4. [DOI] [PubMed] [Google Scholar]
- 10.Huikuri HV, Jokinen V, Syvanne M, Nieminen MS, Airaksinen KE, Ikaheimo MJ, Koistinen JM, Kauma H, Kesaniemi AY, Majahalme S, Niemela KO, Frick MH. Heart rate variability and progression of coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 1999;19:1979–1985. doi: 10.1161/01.atv.19.8.1979. [DOI] [PubMed] [Google Scholar]
- 11.Martin GJ, Magid NM, Myers G, Barnett PS, Schaad JW, Weiss JS, Lesch M, Singer DH. Heart rate variability and sudden death secondary to coronary artery disease during ambulatory electrocardiographic monitoring. Am J Cardiol. 1987;60:86–89. doi: 10.1016/0002-9149(87)90990-8. [DOI] [PubMed] [Google Scholar]
- 12.Airaksinen KE. Autonomic mechanisms and sudden death after abrupt coronary occlusion. Ann Med. 1999;31:240–245. doi: 10.3109/07853899908995886. [DOI] [PubMed] [Google Scholar]
- 13.Bigger JT, Jr, Fleiss JL, Rolnitzky LM, Steinman RC. Frequency domain measures of heart period variability to assess risk late after myocardial infarction. J Am Coll Cardiol. 1993;21:729–736. doi: 10.1016/0735-1097(93)90106-b. [DOI] [PubMed] [Google Scholar]
- 14.Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993;88:927–934. doi: 10.1161/01.cir.88.3.927. [DOI] [PubMed] [Google Scholar]
- 15.Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 59:256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 16.La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 17.La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. Circulation. 1988;78:816–824. doi: 10.1161/01.cir.78.4.816. [DOI] [PubMed] [Google Scholar]
- 18.Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC Study. Atherosclerosis Risk in Communities Study. Am J Epidemiol. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 19.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 20.Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: a review and methodologic critique. Psychol Bull. 1984;96:435–464. [PubMed] [Google Scholar]
- 21.Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321:641–646. doi: 10.1056/NEJM198909073211004. [DOI] [PubMed] [Google Scholar]
- 23.Arad Y, Spadaro LA, Goodman K, Lledo-Perez A, Sherman S, Lerner G, Guerci AD. Predictive value of electron beam computed tomography of the coronary arteries. 19-month follow-up of 1173 asymptomatic subjects. Circulation. 1996;93:1951–1953. doi: 10.1161/01.cir.93.11.1951. [DOI] [PubMed] [Google Scholar]
- 24.Detrano RC, Wong ND, Doherty TM, Shavelle R. Prognostic significance of coronary calcific deposits in asymptomatic high-risk subjects. Am J Med. 1997;102:344–349. doi: 10.1016/s0002-9343(97)00085-5. [DOI] [PubMed] [Google Scholar]
- 25.Witteman JC, Kannel WB, Wolf PA, Grobbee DE, Hofman A, D’Agostino RB, Cobb JC. Aortic calcified plaques and cardiovascular disease (the Framingham Study) Am J Cardiol. 1990;66:1060–1064. doi: 10.1016/0002-9149(90)90505-u. [DOI] [PubMed] [Google Scholar]
- 26.Witteman JC, Kok FJ, van Saase JL, Valkenburg HA. Aortic calcification as a predictor of cardiovascular mortality. Lancet. 1986;2:1120–1122. doi: 10.1016/s0140-6736(86)90530-1. [DOI] [PubMed] [Google Scholar]
- 27.Sloan RP, Korten JB, Myers MM. Components of heart rate reactivity during mental arithmetic with and without speaking. Physiol Behav. 1991;50:1039–1045. doi: 10.1016/0031-9384(91)90434-p. [DOI] [PubMed] [Google Scholar]
- 28.Berntson GG, Quigley KS, Jang J, Boysen ST. An approach to artifact identification: application to heart period data. Psychophysiology. 1990;27:586–598. doi: 10.1111/j.1469-8986.1990.tb01982.x. [DOI] [PubMed] [Google Scholar]
- 29.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;9:1043–1065. [PubMed] [Google Scholar]
- 30.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 31.Sutton-Tyrrell K, Kuller LH, Edmundowicz D, Feldman A, Holubkov R, Givens L, Matthews KA. Usefulness of electron beam tomography to detect progression of coronary and aortic calcium in middle-aged women. Am J Cardiol. 2001;87:560–564. doi: 10.1016/s0002-9149(00)01431-4. [DOI] [PubMed] [Google Scholar]
- 32.Sutton-Tyrrell K, Kuller LH, Matthews KA, Holubkov R, Patel A, Edmundowicz D, Newman A. Subclinical atherosclerosis in multiple vascular beds: an index of atherosclerotic burden evaluated in postmenopausal women. Atherosclerosis. 2002;160:407–416. doi: 10.1016/s0021-9150(01)00591-3. [DOI] [PubMed] [Google Scholar]
- 33.Gallo LC, Matthews KA, Kuller LH, Sutton-Tyrrell K, Edmundowicz D. Educational attainment and coronary and aortic calcification in postmenopausal women. Psychosom Med. 2001;63:925–935. doi: 10.1097/00006842-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74:243–252. doi: 10.4065/74.3.243. [DOI] [PubMed] [Google Scholar]
- 35.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1995;142:889–903. doi: 10.1093/oxfordjournals.aje.a117736. [DOI] [PubMed] [Google Scholar]
- 36.Kuller LH, Matthews KA, Sutton-Tyrrell K, Edmundowicz D, Bunker CH. Coronary and aortic calcification among women 8 years after menopause and their premenopausal risk factors: the healthy women study. Arterioscler Thromb Vasc Biol. 1999;19:2189–2198. doi: 10.1161/01.atv.19.9.2189. [DOI] [PubMed] [Google Scholar]
- 37.Lindheim SR, Legro RS, Bernstein L, Stanczyk FZ, Vijod MA, Presser SC, Lobo RA. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am J Obstet Gynecol. 1992;167:1831–1836. doi: 10.1016/0002-9378(92)91783-7. [DOI] [PubMed] [Google Scholar]
- 38.Saab PG, Matthews KA, Stoney CM, McDonald RH. Premenopausal and postmenopausal women differ in their cardiovascular and neuroendocrine responses to behavioral stressors. Psychophysiology. 1989;26:270–280. doi: 10.1111/j.1469-8986.1989.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 39.Bairey-Merz CN, Kop W, Krantz DS, Helmers KF, Berman DS, Rozanski A. Cardiovascular stress response and coronary artery disease: evidence of an adverse postmenopausal effect in women. Am Heart J. 1998;135:881–887. doi: 10.1016/s0002-8703(98)70050-x. [DOI] [PubMed] [Google Scholar]
- 40.Matthews KA, Stoney CM. Influences of sex and age on cardiovascular responses during stress. Psychosom Med. 1988;50:46–56. doi: 10.1097/00006842-198801000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Devries S, Wolfkiel C, Fusman B, Bakdash H, Ahmed A, Levy P, Chomka E, Kondos G, Zajac E, Rich S. Influence of age and gender on the presence of coronary calcium detected by ultrafast computed tomography. J Am Coll Cardiol. 1995:76–82. doi: 10.1016/0735-1097(94)00342-n. [DOI] [PubMed] [Google Scholar]
- 42.Ferrari AU, Radaelli A, Centola M. Aging and the cardiovascular system. J Appl Physiol. 2003;95:2591–2597. doi: 10.1152/japplphysiol.00601.2003. [DOI] [PubMed] [Google Scholar]
- 43.Tsuji H, Venditti FJ, Jr, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D. Determinants of heart rate variability. J Am Coll Cardiol. 1996;28:1539–1546. doi: 10.1016/s0735-1097(96)00342-7. [DOI] [PubMed] [Google Scholar]
- 44.Colhoun HM, Francis DP, Rubens MB, Underwood SR, Fuller JH. The association of heart-rate variability with cardiovascular risk factors and coronary artery calcification: a study in type 1 diabetic patients and the general population. Diabetes Care. 2001;24:1108–1114. doi: 10.2337/diacare.24.6.1108. [DOI] [PubMed] [Google Scholar]
- 45.Gordon D, Guyton JR, Darnovsky MJ. Intimal alterations in rat aorta induced by stressful stimuli. Lab Invest. 1981;45:14–27. [PubMed] [Google Scholar]
- 46.Hirsch EZ, Maksem JA, Gagen D. Effects of stress and propranolol on the aortic intima of rates. Arthrosclerosis. 1984;4:526. [Google Scholar]
- 47.Kaplan JR, Pettersson K, Manuck SB, Olsson G. Role of sympathoadrenal medullary activation in the initiation and progression of atherosclerosis. Circulation. 1991;84:VI23–VI32. [PubMed] [Google Scholar]
- 48.Manuck SB, Kaplan JR, Adams MR, Clarkson TB. Effects of stress and the sympathetic nervous system on coronary artery atherosclerosis in the cynomolgus macaque. Am Heart J. 1988;116:328–333. doi: 10.1016/0002-8703(88)90110-x. [DOI] [PubMed] [Google Scholar]
- 49.Manuck SB, Kaplan JR, Clarkson TB. Behaviorally-induced heart rate reactivity and atherosclerosis in cynomolgus monkeys. Psychosom Med. 1983;45:95–108. doi: 10.1097/00006842-198305000-00002. [DOI] [PubMed] [Google Scholar]
- 50.Tracey KJ. The inflammatory reflex. Nature. 2002;40:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]


