Abstract
At the heart of the odor recognition process in all animals are G-protein-coupled receptors, which are seven-transmembrane domain proteins that initiate G-protein-mediated signaling cascades when activated by their ligands. Odorant receptors (ORs) are a large, diverse family of proteins with some 80 members in the mosquito Anopheles gambiae. With the assumption that more sensilla on female antennae are tuned to human odors than on male antennae, comparison of specific OR mRNA levels in male and female antennae can provide an indication as to which receptors may be stimulated by host odors. We have used RT PCR and quantitative real-time PCR (qRT PCR) to investigate sex-biased expression levels of 80 A. gambiae ORs in male and female antennae and maxillary palps. On the basis of prevalence of expression in female antennae and on a strong female relative to male expression bias we identified a short list of ORs that are likely involved in host odor recognition by female mosquitoes.
Keywords: Mosquito, Olfaction, Odorant binding protein (OBP), OR, Olfactory sensillum, Trichoid sensillum, mRNA expression, Olfactory organs
1. Introduction
Olfactory stimuli facilitate critical mosquito behaviors, which are often sex specific. Female Anopheles gambiae, the main malarial vector in Sub-Saharan Africa, rely on their sense of smell for host seeking, oviposition and sugar feeding, whereas males respond mostly to plant odors (Foster, 1995; Takken and Knols, 1999). Many field and laboratory studies have shown that female A. gambiae respond to odors emitted from humans to find a blood meal, and wind tunnel experiments and electroantennograms (EAG) have identified a variety of single-chemical compounds in human sweat that stimulate olfactory neurons and attract female mosquitoes (Cork and Park, 1996; Costantini et al., 2001; Dekker et al., 2002; Meijerink et al., 2001; Meijerink and van Loon, 1999; Qiu et al., 2006).
Insect olfactory sensilla located on the main olfactory organs (antennae, maxillary palps, labellum), are distinguished by their morphology, usually contain two olfactory receptor neurons (ORNs) and are specialized to capture specific odors as demonstrated by single-sensillum recordings. Sensilla have pores in their cuticle, through which odor molecules gain access to the sensillar lymph surrounding the ORNs. Several proteins are secreted into the liquid space of the lymph cavity, where the so-called perireceptor events take place (Kaissling, 2001; Pelosi, 1996; Pelosi et al., 2006), but despite recent progress the specificity of odor recognition, transport and degradation in the lymph cavity is still not well understood (Rützler and Zwiebel, 2005). Odor molecules entering the sensillar lymph cavity are believed to be captured by specific carrier proteins (odorant binding proteins, OBPs), which solubilize the odors and transport them to the odorant receptors (ORs) expressed on the dendritic membrane of the ORNs.
The A. gambiae genome contains about 60 putative OBP-encoding genes (Biessmann et al., 2005; Foret and Maleszka, 2006; Vogt, 2002; Xu et al., 2003) and 79 OR genes (Hill et al., 2002), but the vast majority of these proteins have yet to be functionally characterized as to their odor recognition capacity. While the ligand-binding specificity of most Drosophila ORs has been demonstrated using the “empty neuron” system (Hallem et al., 2004b), so far, only AgOR1 and AgOR2 have been tested in this system (Hallem et al., 2004a) and shown to be stimulated by 4-methylphenol (p-cresol) and 2-methylphenol (o-cresol), respectively.
Assuming that more sensilla on female antennae are tuned to human odors than on male antennae, comparison of mRNA levels would provide an indication as to which proteins are most likely involved in host odor recognition because the mRNAs encoding these proteins would represent a larger fraction of the total mRNA population. This reasoning was applied before in identifying several OBPs exhibiting a high female expression bias in A. gambiae antennae, suggesting that they might bind and transport human odor molecules (Biessmann et al., 2005; Justice et al., 2003). However, expression levels of the vast majority of ORs were too low to be detected with any accuracy in these array studies (Biessmann et al., 2005). We report here on the sex-biased expression profiles of 80 ORs in A. gambiae antennae and palps by RT PCR with the goal to identify potential candidate receptors for human odors. Such a comprehensive survey did not exist in the literature and only the expression of ORs 1–4 was previously surveyed in female and male olfactory tissues (Fox et al., 2001).
2. Materials and methods
2.1. RNA isolation and cDNA synthesis
The A. gambiae strain Pink eye was used for all experiments. Total RNA was isolated from hand-dissected antennae or palps from pools of about 500 4–7-day-old sugar-fed females or males using 500 μl of TRIzol Reagent (Gibco BRL Life Technologies, Rockville, MD) and precipitated with isopropanol. RNA was dissolved in 17 μl water and treated with 3U of RQ1 RNase-free DNAse (Promega, Madison, WI) for 1 h at 37°C. After adding 2.4 μl stop solution, the enzyme was inactivated for 10 min at 70 °C. About 3 μg of each RNA were converted to cDNA in 20 μl reactions using 0.5 μg oligo dT primer, 0.5mM dNTPs, 10mM DTT, first strand synthesis buffer and 70U of SuperscriptII reverse transcriptase (Invitrogen, Carlsbad, CA) by incubating for 5 min at 65 °C (without enzyme) followed by incubation for 4 h at 42 °C after enzyme addition. Reactions were terminated at 70 °C for 15 min and aliquots were used in PCR reactions.
2.2. RT PCR
Matching primer sets with matching melting temperatures between 58 and 67 °C (Sigma-Genosys, The Woodland, TX) were designed such that they were positioned in two different exons to distinguish products generated from cDNA from those of residual genomic DNA or unprocessed primary transcripts (see Supplemental Table 1) or, for two of them, straddling an exon–intron boundary. Expected fragments from cDNA were between 100 and 200 bp, while those from genomic DNA were mostly >200 bp. RT PCR was done in 25 μl containing 200nM of forward and reverse primers, 200 μM dNTPs, 2.5U Hotmaster Taq polymerase (Eppendorf AG, Hamburg, Germany) in 1 × buffer provided by the manufacturer, and, typically, 1 μl of a 1:20 dilution of the above cDNA reactions (~40 ng of cDNA). For each preparation, the ribosomal protein S7 (RpS7) cDNA was titrated by qRT PCR and served as an internal control for equal cDNA input. Based on this quantification, the appropriate dilution of male antennal cDNA was chosen for the subsequent PCR reactions. PCR reactions were initially denatured at 94°C for 3 min, followed by 30-s denaturation at 94 °C, annealing at 55 °C for 30 s, and a 1-min extension at 72 °C for 27–40 cycles. Products were loaded and electrophoresed in a 2.5% agarose gel in TAE and stained with ethidium bromide.
2.3. Quantitative real-time PCR (qRT PCR)
For quantitative RT PCR, a BioRad iCycler iQ™ Real-Time PCR cycler (BioRad, Hercules, CA) was used with SYBR Green as detection reagent. Reactions were performed in 96-well microtiter plates in 20 μl volumes each containing 200 μM dNTPs, 0.1 mg/ml BSA, 0.1 × SYBR Green (10,000 × from Invitrogen, Carlsbad, CA) and 0.5U Hotmaster Taq polymerase (Eppendorf AG, Hamburg, Germany) in 1 × buffer provided by the manufacturer, 200nM of each primer pair and 1 μl of a 1:20 dilution of the cDNA. Because expression of some of the ORs was very low in male antennae, a five-fold higher concentration of male antennal cDNA was used in these reactions. The qRT PCR reactions were done using the following program: 3 min at 94 °C followed by 40 cycles with 30 s at 94 °C, 30 s at 55 °C and 45 s at 65 °C. A melting curve analysis was done at the end of each reaction to ensure quality of the amplified product. The competitive threshold cycle method was used for quantification. In every microtiter plate, a control curve was generated with each primer pair, and each sample point was done in three technical replicates. Determinations of OR mRNA abundance were based on three independent biological replicates. The obtained values were averaged and normalized for each preparation with the concentration of ribosomal protein S7 (RpS7) mRNA.
3. Results
There is about a three-fold difference in the number of trichoid sensilla, which are mainly involved in odor detection, between A. gambiae female (629 located in a total of 13 antennal segments) and male (227 mostly located in the two terminal segments of the male antenna) (McIver, 1982). To identify those A. gambiae ORs that are most likely involved in responding to human odors, we first surveyed their levels of expression in male and female antennae. We reasoned that female antennae contain more sensilla that are tuned to human odors than male antennae and thus, a female-biased expression would be a first level of characterization of those ORs. This selection could then be applied to narrow down the number of potentially interesting ORs from the 80 encoded in the genome to about a dozen to be used in further studies.
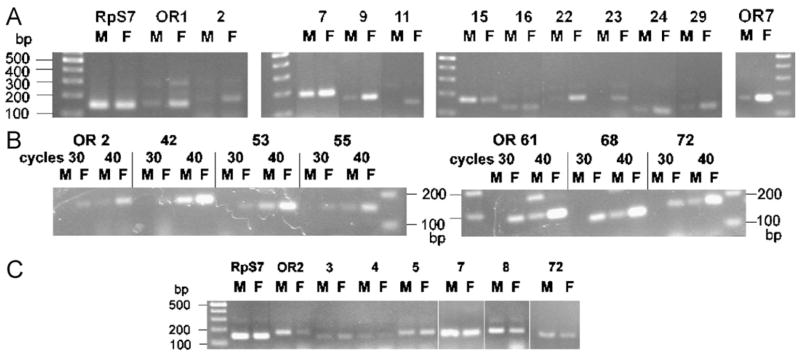
As a first approach we used RT PCR to compare steady-state mRNA levels in male and female antennae using primer sets that give rise to different size fragments from cDNA and any genomic DNA contamination (or presence of unprocessed primary transcripts). The ribosomal protein S7 (RpS7) cDNA was titrated by qRT PCR for each preparation and served as an internal control for equal cDNA input. Examples of ethidium bromide-stained 2.5% agarose gels are shown in Fig. 1A (35 amplification cycles) and Fig. 1B (30 and 40 amplification cycles). These gels show that, as expected, OR7 is the most abundantly expressed receptor in male and female antennae with a female bias. The A. gambiae OR7 (Melo et al., 2004; Pitts et al., 2004) is believed to be the ortholog to the Drosophila melanogaster OR83b, which is abundantly expressed in Drosophila ORNs (Larsson et al., 2004) and forms heterodimers with conventional ORs (Nakagawa et al., 2005; Neuhaus et al., 2005). Interestingly, OR83b-deficient flies are anosmic (Larsson et al., 2004), suggesting an obligatory function in olfaction in Drosophila. In the examples in Figs. 1A and B, while OR15 was seen to have a male bias and OR16 to be about equally expressed in female and male antennae, the other ORs exhibited various degrees of female bias.
Fig. 1.
Representative examples of ethidium bromide-stained 2.5% agarose gels of RT PCR products amplified from male (M) or female (F) antennal (A, B) and palp (C) cDNAs. Thirty-five amplification cycles were used in (A and C) except in the last panel of (A), which shows amplification of OR7 with 30 cycles to demonstrate its strong female bias. Thirty and 40 cycles were used in side-by-side comparison for selected ORs in (B) to show the presence of low level expression in male antennae. Prior to RT PCR, cDNA populations were normalized by qRT PCR to the amount of RpS7 expression levels to assure equal input.
The RT PCR results of all 80 ORs are compiled in Table 1, from which the following conclusions can be drawn (Fig. 2). Different ORs are expressed at widely varying levels in A. gambiae antennae, with eighteen being undetectable in male or female antennae under the amplification conditions employed in these experiments. Four ORs (OR16, 26, 30, 33) were equally abundant in male and female antennae and three (OR13, 15, 20) exhibited a male bias. Of the remaining ORs exhibiting various degrees of female-biased expression, 23 had a strong female bias with 14 of those being expressed very abundantly in female antennae (ORs 7, 9, 22, 29, 42, 53, 61, 66, 67, 68, 70, 71, 72, 76) [intensity on stained gels equal or greater than ++(+); Table 1 and Fig. 2]. Thirty-one ORs exhibited a less pronounced yet convincing female bias with one of them being abundantly expressed in female antennae (OR32).
Table 1.
RT PCRs for OR expression in antennae and palps
| OR no. | FA to MA ratio | FA abundance | FP to MP ratio | Palp abundance |
|---|---|---|---|---|
| 1 | F>M | + + | U | U |
| 2 | F≫M (U) | + + | F≪M | + +( + ) |
| 3 | U | U | F= M | +( + ) |
| 4 | U | U | F<M | + |
| 5 | U | U | F = M | + + |
| 6 | U | U | U | U |
| 7 | F≫M | + + + + | F=M | + + + + |
| 8 | U | U | F<M | + + +( + ) |
| 9 | F≫M | + + +( + ) | U | U |
| 10 | U | U | U | U |
| 11 | F≫M (U) | + + | F = M | ( + ) |
| 12 (19/50) | F>M (U) | + | U | U |
| 13 | F<M | ( + ) | U | U |
| 14 | U | U | U | U |
| 15 | F<M ( + + + ) | +( + ) | F<M | + + |
| 16 | F = M | +( + ) | U | U |
| 17 | F>M (U) | + | U | U |
| 18 | F>M | + | U | U |
| 19 (12/50) | F>M (U) | + | U | U |
| 20 | F<M ( + ) | U | F>M | + |
| 21 | F>M | +( + ) | F = M | + + |
| 22 | F≫M | + + + | U | U |
| 23 | F≫M (U) | +( + ) | U | U |
| 24 | F>M | + + | U | U |
| 25 | F>M (U) | +( + ) | U | U |
| 26 | F = M | ( + ) | U | U |
| 27 | F≫M (U) | +( + ) | U | U |
| 28 | U | U | F = M | + +( + ) |
| 29 | F≫M | + +( + ) | U | U |
| 30 | F = M | ( + ) | U | U |
| 31 | F>M | ( + ) | U | U |
| 32 | F>M | + +( + ) | U | U |
| 33 | F = M | ( + ) | U | U |
| 34 | F≫M | +( + ) | U | U |
| 35 | F≫M | +( + ) | U | U |
| 36 | F≫M | +( + ) | U | U |
| 37 | U | U | U | U |
| 38 | F>M | ( + ) | U | U |
| 39 | F≫M | + + | U | U |
| 40 | U | U | U | U |
| 41 | F>M | +( + ) | U | U |
| 42 | F≫M | + + + | U | U |
| 43 | F>M | ( + ) | U | U |
| 44 | F>M | ( + ) | U | U |
| 45 | F≫M | + + | U | U |
| 46 | F>M | + | U | U |
| 47 | F>M | ( + ) | U | U |
| 48 | F>M | ( + ) | U | U |
| 49 | U | U | U | U |
| 50 (12/19) | F>M (U) | + | U | U |
| 51 | F>M | +( + ) | U | U |
| 52 | U | U | U | U |
| 53 | F≫M | + + + | U | U |
| 54 | F>M | +( + ) | U | U |
| 55 | F>M | + + | U | U |
| 56 | F>M | + + | U | U |
| 57 | F>M | +( + ) | U | U |
| 58 | U | U | U | U |
| 59 | F>M | + | U | U |
| 60 | F>M | +( + ) | U | U |
| 61 | F≫M | + +( + ) | U | U |
| 62 | F>M | +( + ) | U | U |
| 63 | U | U | U | U |
| 64 | U | U | U | U |
| 65 | U | U | U | U |
| 66 | F≫M | + +( + ) | U | U |
| 67 | F≫M | + +( + ) | U | U |
| 68 | F≫M | + + + | U | U |
| 69 | F>M | + | U | U |
| 70 | F≫M | + +( + ) | U | U |
| 71 | F≫M | + +( + ) | U | U |
| 72 | F≫M | + + + | F<M | + + |
| 73 | F>M | + | U | U |
| 74 | F>M | ( + ) | U | U |
| 75 | F>M | ( + ) | U | U |
| 76 | F≫M | + +( + ) | U | U |
| 77 | U | U | U | U |
| 78 | F>M | + + | U | U |
| 79 | U | U | U | U |
| 80 | U | U | F<M | + |
Summary of RT PCR analyses of OR expression in female (FA) and male (MA) antennae and in female (FP) and male (MP) palps. The first column lists the OR numbers according to the Ensemble A. gambiae website http://www.ensembl.org/Anopheles_gambiae/index.html. The OR listed as OR80 has the transcript number ENSANGT00000001823. ORs 12, 19 and 50 are indistinguishable. The second column assigns the estimated ratio of expression in FA vs. MA as judged from the intensity of the ethidium bromide-stained bands of the size predicted from the fully processed mRNA. ORs that have a very strong female bias are designated F≫M, those with a moderate female bias are shown as F>M, male-biased ORs are shown as F<M, ORs showing equal expression in female and male antennae are shown by F = M and undetectable expression is indicated by U. Designations in parentheses (e.g. (U)) indicate that expression of this OR was undetectable in male antennae. The third column shows the expression levels in female antennae on a scale from + + + + (strong) to ( + ) (very weak). The fourth column indicates female or male bias of expression in palps and column five shows relative estimated abundance in palps.
Fig. 2.
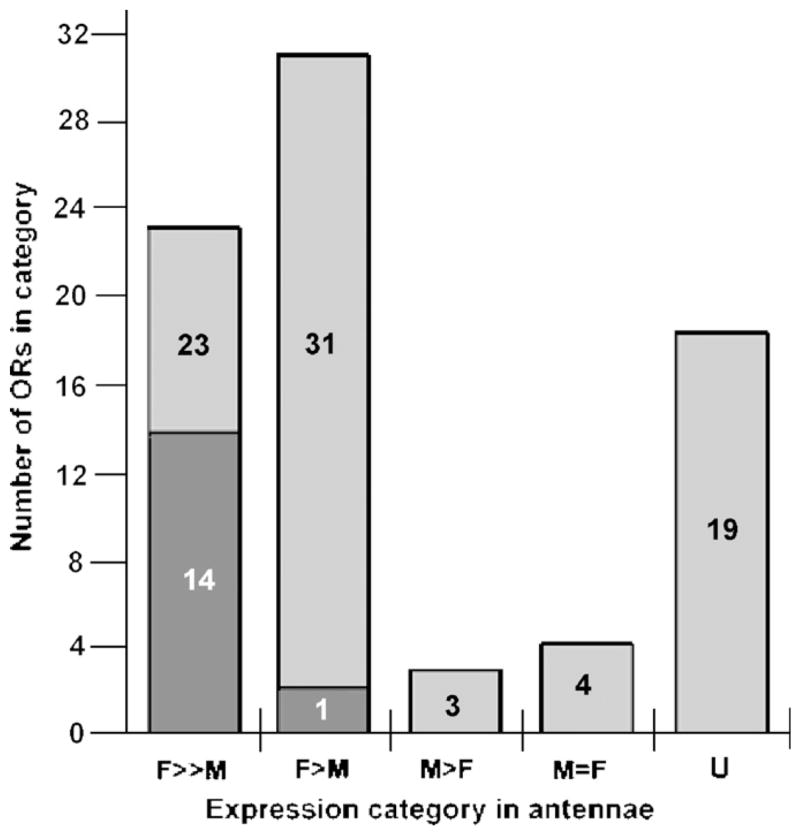
Bar graph summarizing sex-biased expression of 80 ORs in antennae as based on ethidium bromide-stained gels of RT PCR products from cDNA preparation that had been normalized to RpS7 expression levels. Twenty-three ORs have a high female bias (F≫M) with 14 of those (bar portion indicated with darker shading; see column 3 of Table 1) being abundantly expressed in female antennae [intensity equal or greater than + + ( + )]; 31 ORs have a moderate female bias (F>M) with one of those being highly expressed in female antennae; three ORs have a male bias (M>F); four ORs are equally expressed in male and female antennae (M = F); and 19 were undetectable by our PCR methods in either male or female antennae even with 40 amplification cycles.
Real time RT PCR was performed to confirm and quantify sex difference of expression for some representative ORs. The selected ORs showed the following female: male antennal expression ratios (normalized to RpS7) ± STD: OR1: 19.6±2.1; OR2: 12.6±6.5; OR7: 12.6±4.5; OR42: 11.0±5.8; OR55: 11.9±3.0; OR61: 19.4±7.0; OR68: 15.3±7.8; OR72: 12.3±5.1. The strong female bias of these ORs was also obvious from the ethidium-stained gels, especially with lower amplification cycles (Figs. 1A, B). However, these ORs were not completely absent from male antennae, as amplification with 40 cycles clearly produced the correct sizes of PCR products (Fig. 1B).
Male mosquitoes have the same types of sensilla as females, however, these are restricted to the terminal two flagellar segments (McIver, 1982). The remainder of the male flagellum serves as a very sensitive sound detector (Göpfert and Robert, 2000). To evaluate this on a molecular level, we measured the abundance of OR7, which is expressed in most olfactory neurons (Melo et al., 2004; Pitts et al., 2004). After removing the last two segments from ~150 male antennae, RNA was extracted and converted to cDNA as above. When OR7 mRNA levels were determined by qRT PCR in these “tipless” antennae and compared to those from whole male antennae, we found that the RpS7-normalized values for OR7 were reduced by a factor of 144 (data not shown), confirming the previous morphological observations. Thus, OR7 mRNA levels can serve as an approximate measurement of sensillar density.
The maxillary palps are an important chemosensory tissue but harbor considerably fewer sensilla than antennae, (McIver, 1982). Our RT PCR data show (Fig. 1C and Table 1) that some ORs (ORs 2, 7, 11, 15, 20, 21, 72) are not only expressed in antennae but also on palps, in contrast to the majority of the other ORs, which are not expressed in palps at a detectable level. Interestingly, expression of ORs 3, 4, 5, 8, 28 and 80 was only detected on palps but not antennae.
4. Discussion
Based on our results, we are identifying the subset of 14 ORs (including OR7) that are highly expressed in female antennae and have a strong female/male bias as the strongest candidate receptors for human odor detection by female mosquitoes, with the remaining nine ORs of the F≫M group being a second set of candidates for a similar function. We also note that this subset includes OR1 and OR2 that are stimulated by cresols, components of sweat. Whether these ORs are in any way correlated to the expression of OBPs that exhibit a similar female bias (Biessmann et al., 2005) will have to be investigated by co-localization studies.
The 31 ORs that are found to have a limited female bias of mRNA accumulation may serve olfactory functions that are common in male and female mosquitoes such as detection of odors emitted by floral food sources. Although no electrophysiological data are yet available for male A. gambiae, sensilla on the antennae of male Aedes aegypti are sensitive to many of the same compounds that attract females (Davis, 1977). This sensitivity may bring the males close to the females that are feeding on a warm-blooded host and increase their chance of mating.
The three ORs with a male expression bias as well as the four ORs with equal levels of expression in male and female antennae are possible candidates for binding of other odors that are preferentially recognized my male mosquitoes. The validity of these hypotheses will require experimental verification.
The interpretation of the observed quantitative differences between levels of specific OR mRNA expression in female and male antennae requires consideration, especially whether it is fair to compare gene expression between whole antennae. If we assume that the mainly non-sensory part of the male antenna expresses the same amount of RpS7 as the last two sensory segments, this would lead to an under-estimation of OR expression in male antennae when normalized to RpS7. However, we do not know to what extent this is the case because the cuticular part of the proximal segments of the male antenna may contain few live cells. Alternatively, if we assume that levels of mRNA are based solely on numbers of trichoid sensilla present on female and male antennae (629 vs. 227; McIver, 1982), we should expect an a priori ground state of mRNA accumulation that would be approximately three times higher in female than in male antennae for all ORs that recognize common odors in male and female mosquitoes. In fact, this is about what we are observing for the majority of ORs (31 ORs designated F>M; Fig. 2). For OR7, we determined by qRT PCR a 12.6-fold higher level in female than male antennae, indicating that a greater proportion of sensilla on female antennae express OR7 than on male antennae. We speculate that perhaps OR7 is more commonly associated as a dimerization partner with those ORs that are abundantly expressed in females.
The maxillary palps harbor considerably fewer sensilla than antennae, (McIver, 1982). As in other insects, they are believed to specialize in taste reception and in some mosquitoes contain CO2-sensitive sensilla (reviewed in (Takken and Knols, 1999). In fact, ORNs on palps of A. aegypti (Grant and O’Connell, 1996; Grant et al., 1995) and A. gambiae (Lu et al., 2007) can be stimulated with CO2 as well as with 1-octen-3-ol. Interestingly, Lu et al. (2007) reported that the CO2-sensitive ORNs express three gustatory receptors, while the 1-octen-3-ol-sensitive ORN expresses OR8, which we find to be highly expressed in palps (with a slight male bias; Table 1) but undetectable in antennae. The male-biased expression of OR8 cannot be explained at this time because of the absence of behavioral and electrophysiological data comparing responses in females vs. males to 1-octen-3-ol, which could be used as guide. It should be also pointed out that different mosquito species respond to different host odor cues and that observations with different mosquito species may not be applicable to A. gambiae. In fact, although 1-octen-3-ol elicits an electrophysiological response in antennae (Cork and Park, 1996; Qiu et al., 2006; van den Broek and den Otter, 1999) and palps (Lu et al., 2007) of A. gambiae, it has no attracting effect on females in wind tunnel experiments (Takken et al., 1997) and does not cause significant increases in trap catches in the field (Njiru et al., 2006), suggesting that 1-octen-3-ol (a compound emitted by cattle ruminants) may not be a major host-finding cue for this anthropophilic mosquito species.
A third, broadly tuned ORN, which expresses OR28, was also identified on A. gambiae palps (Lu et al., 2007). Again, we find OR28 to be highly expressed in palps (equally in females and males; Table 1) but undetectable in antennae. Four other ORs (ORs 3–5 and 80) that we found to be palp-specific will be worth pursuing. However, their expression levels appear to be lower than those of OR8 and 28.
In our study, we also found seven ORs (ORs 2, 7, 11, 15, 20, 21, 72) to be expressed in palps as well as in antennae. In previous experiments, we had found also some overlap of OBP expression in antennae and palps, and about half of the OBPs expressed in antennae, which included those with the highest expression levels, were also expressed in palps (Biessmann et al., 2005). Our new data support the idea that both tissues may have some overlapping function or at least use the same proteins for both types of chemoreception. Perhaps the taste function of palps becomes important after the mosquito has landed on the vertebrate host by sensing the same chemicals at short range and, thus, enhancing the antennal stimulation and causing the probing response.
In our study, we did not analyze OR expression in the proboscis, which is also a part of the mosquito chemosensory system, but a RT PCR survey (Kwon et al., 2006) found 16 ORs to be expressed in the proboscises of both sexes, while nine could only be identified in females. We found the vast majority of these 25 ORs (all except for OR3, 4, 6, 14, and 28) to be also expressed in antennae. Interestingly, of the nine ORs that were reported to be expressed only in female proboscises, four (OR39, 68, 70 and 71) also had a strong female bias in antennae in our survey (classified as F≫M), suggesting a role in host odor detection by females.
An OR expression study in olfactory tissues has recently been published for A. aegypti reporting comparable overlapping expression patterns but also tissue specificities (Bohbot et al., 2007). In some cases, true orthologous ORs may be identified by sequence alone, but, in most cases, classification will have to await functional analyses. We note that the orthologs AgOR8 and AaOR8 both appear to have palp-specific expression, but only AgOR22, which is expressed with a strong female bias in A. gambiae antennae, has an A. aegypti ortholog (AaOR22P) with a similar sex bias.
Supplementary Material
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ibmb.2007.11.008.
Acknowledgments
This work was supported by a Fulbright Visiting Scientist award to K.I. and grants from the General Secretariat of Science and Technology, Greek Ministry of Development (Greece-USA 052 to K.I.) and the US Public Health Service (AI051485 to H. B.). We thank Luc Swevers for critical reading of the manuscript. We also acknowledge the assistance of Diana Le, Adam Draper and Charné Moore.
References
- Biessmann H, Nguyen QK, Le D, Walter MF. Microarray-based survey of a subset of putative olfactory genes in the mosquito Anopheles gambiae. Insect Mol Biol. 2005;14:575–589. doi: 10.1111/j.1365-2583.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- Bohbot J, Pitts RJ, Kwon HW, Rützler M, Robertson HM, Zwiebel LJ. Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol Biol. 2007;16:525–537. doi: 10.1111/j.1365-2583.2007.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork A, Park KC. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med Vet Entomol. 1996;10:269–276. doi: 10.1111/j.1365-2915.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Costantini C, Birkett MA, Gibson G, Ziesmann J, Sagnon NF, Mohammed HA, Coluzzi M, Pickett JA. Electroantennagram and behavioural response of the malaria vector Anopheles gambiae to human-specific sweat components. Med Vet Entomol. 2001;15:259–266. doi: 10.1046/j.0269-283x.2001.00297.x. [DOI] [PubMed] [Google Scholar]
- Davis EE. Response of the antennal receptors of the male Aedes aegypti mosquito. J Insect Physiol. 1977;23:613–617. [Google Scholar]
- Dekker T, Steib B, Cardé RT, Geier M. L-lactic acid: a human-signifying host cue for the anthropophilic mosquito Anopheles gambiae. Med Vet Entomol. 2002;16:91–98. doi: 10.1046/j.0269-283x.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- Foret S, Maleszka R. Function and evolution of a gene family encoding odorant binding-like proteins in a social insect, the honey bee (Apis mellifera) Genome Res. 2006;16:1404–1413. doi: 10.1101/gr.5075706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WA. Mosquito sugar feeding and reproductive energetics. Ann Rev Entomol. 1995;40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- Fox AN, Pitts RJ, Robertson HM, Carlson JR, Zwiebel LJ. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc Natl Acad Sci USA. 2001;98:14693–14697. doi: 10.1073/pnas.261432998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert MC, Robert D. Nanometre-range acoustic sensitivity in male and female mosquitoes. Proc R Soc Lond B. 2000;267:453–457. doi: 10.1098/rspb.2000.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AJ, O’Connell RJ. Electrophysiological responses from receptor neurons in mosquito maxillary palps sensilla. In: Hildebrand JG, editor. Olfaction in Mosquito–Host Interactions. Wiley; Chichester: 1996. pp. 233–248. [DOI] [PubMed] [Google Scholar]
- Grant AJ, Wigton BE, Aghajanian JG, O’Connell RJ. Eletrophysiological response of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J Comp Physiol A. 1995;177:389–396. doi: 10.1007/BF00187475. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Fox AN, Zwiebel LJ, Carlson JR. Mosquito receptor for human-sweat odorant. Nature. 2004a;427:212–213. doi: 10.1038/427212a. [DOI] [PubMed] [Google Scholar]
- Hallem EA, Ho MG, Carlson DA. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004b;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, Cravchik A, Collins FH, Robertson HM, Zwiebel LJ. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–178. doi: 10.1126/science.1076196. [DOI] [PubMed] [Google Scholar]
- Justice RW, Dimitratos S, Walter MF, Woods DF, Biessmann H. Sexual dimorphism of antennal gene expression in the malaria vector Anopheles gambiae. Ins Mol Biol. 2003;12:581–594. doi: 10.1046/j.1365-2583.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- Kaissling KE. Olfactory perireceptor and receptor events in moths: a kinetic model. Chem Senses. 2001;26:125–150. doi: 10.1093/chemse/26.2.125. [DOI] [PubMed] [Google Scholar]
- Kwon HW, Lu T, Rützler M, Zwiebel LJ. Olfactory responses in a gustatory organ of the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2006;103:13526–13531. doi: 10.1073/pnas.0601107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encoes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, van Loon JJA, Takken W, Carlson JR, Zwiebel LJ. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver SB. Sensilla of mosquitoes (Diptera: Culicidae) J Med Entomol. 1982;19:489–535. doi: 10.1093/jmedent/19.5.489. [DOI] [PubMed] [Google Scholar]
- Meijerink J, van Loon JJA. Sensitivities of antennal olfactory neurons of the malaria mosquito, Anopheles gambiae, to carboxylic acids. J Insect Physiol. 1999;45:365–373. doi: 10.1016/s0022-1910(98)00135-8. [DOI] [PubMed] [Google Scholar]
- Meijerink J, Braks MAH, van Loon JJA. Olfactory receptors on the antennae of the malaria mosquito Anopheles gambiae are sensitive to ammonia and other sweat-borne components. J Insect Physiol. 2001;47:455–464. doi: 10.1016/s0022-1910(00)00136-0. [DOI] [PubMed] [Google Scholar]
- Melo ACA, Rützler M, Pitts RJ, Zwiebel LJ. Identification of a chemosensory receptor from the yellow fever mosquito, Aedes aegypti, that is highly conserved and expressed in olfactory and gustatory organs. Chem Senses. 2004;29:403–410. doi: 10.1093/chemse/bjh041. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Sakurai T, Nishioka T, Touhara K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science. 2005;307:1638–1642. doi: 10.1126/science.1106267. [DOI] [PubMed] [Google Scholar]
- Neuhaus EM, Gisselmann G, Zhang W, Dooley R, Störtkuhl K, Hatt H. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat Neurosci. 2005;8:15–17. doi: 10.1038/nn1371. [DOI] [PubMed] [Google Scholar]
- Njiru BN, Mukabana WR, Takken W, Knols BG. Trapping of the malaria vector Anopheles gambiae with odour-baited MM-X traps in semi-field conditions in Western Kenya. Malaria J. 2006;5:39, 8. doi: 10.1186/1475-2875-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelosi P. Perireceptor events in olfaction. J Neurobiol. 1996;30:3–19. doi: 10.1002/(SICI)1097-4695(199605)30:1<3::AID-NEU2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Pelosi P, Zhou JJ, Ban LP, Calvello M. Soluble proteins in insect chemical communication. Cell Mol Life Sci. 2006;63:1658–1676. doi: 10.1007/s00018-005-5607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Fox AN, Zwiebel LJ. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 2004;101:5058–5063. doi: 10.1073/pnas.0308146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu YT, van Loon JJA, Takken W, Meijerink J, Smid HM. Olfactory coding in antennal neurons of the malaria mosquito, Anopheles gambiae. Chem Senses. 2006;31:845–863. doi: 10.1093/chemse/bjl027. [DOI] [PubMed] [Google Scholar]
- Rützler M, Zwiebel LJ. Molecular biology of insect olfaction: recent progress and conceptual models. J Comp Physiol A. 2005;191:777–790. doi: 10.1007/s00359-005-0044-y. [DOI] [PubMed] [Google Scholar]
- Takken W, Knols BGJ. Odor-mediated behavior of afrotropical malaria mosquitoes. Annu Rev Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- Takken W, Dekker T, Wijnholds GY. Odor-mediated flight behavior of Anopheles gambiae Giles sensu stricto and An. stephensi Liston in response to CO2, acetone, and 1-octen-3-ol (Diptera: Culicidae) J Ins Behav. 1997;10:395–407. [Google Scholar]
- van den Broek IVF, den Otter CJ. Olfactory sensitivities of mosquitoes with different host preferences (Anopheles gambiae s.s., An arabiensis, An quadriannulatus, An m atroparvus) to synthetic host odours. J Ins Physiol. 1999;45:1001–1010. doi: 10.1016/s0022-1910(99)00081-5. [DOI] [PubMed] [Google Scholar]
- Vogt RG. Odorant binding protein homologues of the malaria mosquito Anopheles gambiae; possible orthologues of the OS-E and OS-F OBPs of Drosophila melanogaster. J Chem Ecol. 2002;28:2371–2376. doi: 10.1023/a:1021009311977. [DOI] [PubMed] [Google Scholar]
- Xu PX, Zwiebel LJ, Smith DP. Identification of a distinct family of genes encoding atypical odorant-binding proteins in the malaria vector mosquito, Anopheles gambiae. Insect Mol Biol. 2003;12:549–560. doi: 10.1046/j.1365-2583.2003.00440.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ibmb.2007.11.008.