Abstract
Purpose
Prolonged contact of opposite mucosal surfaces, which occurs on the ocular surface, oral cavity, reproductive tract, and gut, requires a specialized apical cell surface that prevents adhesion. The purpose of this study was to evaluate the contribution of mucin O-glycans to the antiadhesive character of human corneal–limbal epithelial (HCLE) cells.
Methods
Mucin O-glycan biosynthesis in HCLE cells was disrupted by metabolic interference with benzyl-α-GalNAc. The cell surface mucin MUC16 and its carbohydrate epitope H185 were detected by immunofluorescence and Western blot. HCLE cell surface features were assessed by field emission scanning electron microscopy. Cell–cell adhesion assays were performed under static conditions and in a parallel plate laminar flow chamber.
Results
Benzyl-α-GalNAc disrupted the biosynthesis of O-glycans without affecting apomucin biosynthesis or cell surface morphology. Static adhesion assays showed that the apical surface of differentiated HCLE cells expressing MUC16 and H185 was more antiadhesive than undifferentiated HCLE cells, which lacked MUC16. Abrogation of mucin O-glycosylation in differentiated cultures with benzyl-α-GalNAc resulted in increased adhesion of applied corneal epithelial cells and corneal fibroblasts. The antiadhesive effect of mucin O-glycans was further demonstrated by fluorescence video microscopy in dynamic flow adhesion assays. Cationized ferritin labeling of the cell surface indicated that anionic repulsion did not contribute to the antiadhesive character of the apical surface.
Conclusions
These results indicate that epithelial O-glycans contribute to the antiadhesive properties of cell surface–associated mucins in corneal epithelial cells and suggest that alterations in mucin O-glycosylation are involved in the pathology of drying mucosal diseases (e.g., dry eye).
Cell surface–associated mucins, a group of high-molecular-weight glycoproteins lining the apical cell surface on mucosal tissues, constitute a major component of the glycocalyx on epithelial cells.1,2 To date, 10 members of this class of molecules, designated MUC, have been described, all being type I membrane proteins with single transmembrane domains.3 The human ocular surface epithelia express at least three membrane-associated mucins, MUC1, -4, and -16.4 Structurally, they are defined by the presence of central tandem repeats of amino acids rich in serine, threonine, and proline residues, and by their extensive O-glycosylation. The amount of O-glycans varies depending on the tissue in which they are expressed (e.g., representing up to 50% of the mucin mass in the mammary gland and up to 80% in the pancreas).5
Alteration in mucin-type O-glycosylation has been demonstrated in patients with drying diseases such as dry eye6,7 and dry mouth.8,9 In these diseases—frequently chronic and characterized by adhesiveness of the apical surface epithelia—lubricants are usually applied to reduce epithelial damage to the cell surface by abrasive stress. Because of their large extracellular domain, which extends up to 500 nm above the plasma membrane, cell surface mucins have been shown to confer an antiadhesive character to cell membranes.10,11 Preclusion of adhesion has been shown to depend on the level of mucin expression, and the length and number of tandem repeats that extend into the pericellular space. In this regard, overproduction of cell surface mucin by transfecting HBL-100 human mammary epithelial cells and A375 human melanoma cells with full-length complementary DNA encoding MUC1 has been shown to strongly reduce cellular aggregation.12 Similarly, transfection of A375 human melanoma cells or murine L929 cells with a series of recombinant cDNA containing increasing lengths of cell surface-associated mucins shows a decreasing number of cell–cell interactions.13,14 Because of the extensive glycosylation of mucins, it has been proposed that O-glycans may also contribute to the antiadhesive character of mucins by either preserving the extended, rigid structure of the mucin molecule or by charge repulsion, due to the abundance of negatively charged sialic acids.11 Removal of sialic acid by neuraminidase, however, has yielded controversial results in aggregation assays, showing that sialic acid residues are either not required for the antiadhesive effect of the mucin or partially contribute to modulating cell adhesion.12,13
To test whether mucin O-glycans and negative charges prevent apical cell–cell interactions, we have disrupted the biosynthesis of cell surface O-glycans in vitro by metabolic interference using benzyl-α-GalNAc.15,16 This chemical primer competes with endogenous substrates for elaboration of the core GalNAc residue; therefore, cells treated with this compound express truncated mucin-type O-linked glycans.17 Using static and dynamic flow assays, we showed that cell surface O-glycans contribute to the prevention of adhesion on the apical surface of normal, differentiated corneal epithelial cells and that this effect is not mediated by negative charges.
Methods
Cell Culture
The role of O-glycans as disadhesive molecules at the apical epithelial surface was studied using a telomerase-immortalized human corneal–limbal epithelial (HCLE) cell line that expresses glycosylated cell surface–associated mucins similar to those in native corneal epithelium.18 Apical cells in HCLE cultures produce the terminal carbohydrate epitope H185, an O-acetyl sialic acid, that is carried by the cell surface-associated mucin MUC16 in terminally differentiated cells.19,20
HCLE cells were plated at a density of 5 × 104 cells/cm2 and grown on one-well slides to confluence in keratinocyte serum-free medium (Invitrogen-Gibco, Rockville, MD), supplemented with 25 μg/mL bovine pituitary extract (BPE), 0.2 nM epidermal growth factor (EGF), and 0.4 mM CaCl2, at 37°C in a 5% carbon dioxide atmosphere (SFM condition). Thereafter, for experiments requiring stratified cultures that produce glycosylated cell surface–associated mucins on the apical cell surface, HCLE cells were switched to DMEM/Ham’s F-12 medium (50:50; Mediatech, Inc.; Herndon, VA) with high calcium (1 mM CaCl2) supplemented with 10% calf serum and 10 ng/mL EGF for 7 days (Serum condition). To inhibit mucin O-glycosylation, 2 mM benzyl-α-GalNAc (BG) in dimethyl sulfoxide was added to HCLE cells grown for 7 days in serum-containing media (Serum+BG condition) as reported elsewhere.21,22
In control experiments, primary cultures of human corneal fibroblasts, which do not express mucin O-glycans, were grown in DMEM/Ham’s F-12 medium supplemented with 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO) in the absence or presence of 2 mM benzyl-α-GalNAc.
Immunofluorescence Microscopy
The cell surface–associated mucin MUC16 and the H185 carbohydrate epitope on MUC16 were localized on HCLE cell surfaces by immunofluorescence microscopy, as previously described.19,20 Briefly, cell culture slides were incubated for 1 hour at room temperature with the MUC16 (OC125, 1:50; Dako Corp., Carpinteria, CA) or H185 primary antibodies, followed by a fluorescein-conjugated donkey anti-mouse IgG secondary antibody (1:50; Jackson ImmunoResearch; West Grove, PA). Slides were then coverslipped with mounting medium (Vectashield; Vector Laboratories, Burlingame, CA) plus propidium iodide, to localize the position of the cell nuclei in the culture. Primary antibodies were omitted from sections used as the negative control.
Scanning Electron Microscopy
Scanning electron microscopy was used to visualize and compare the ultrastructural features of the surface of HCLE cells grown under different conditions (SFM, Serum, Serum+BG). HCLE cell cultures were washed three times in phosphate-buffered saline (PBS) and fixed in half-strength Karnovsky’s fixative for 1 hour. They were then rinsed in PBS, dehydrated through graded ethanols, critical point dried (Autosamdri 795 Supercritical Point Dryer; Tousimis, Rockville, MD), mounted on specimen holders, and sputter coated with carbon in an ion beam coater (model 681; Gatan, Pleasanton, CA). Images were acquired via a field emission scanning electron microscope (JSM-7401F; JEOL Ltd., Tokyo, Japan).
Electrophoresis and Western/Lectin Blot
Protein was extracted from HCLE cells by using 2% sodium dodecyl sulfate (SDS) plus a protease inhibitor cocktail (Complete; Roche Biochemical, Indianapolis, IN). Protein concentration was determined with the BCA protein assay reagent kit (Pierce, Rockford, IL). Proteins were diluted in Laemmli buffer and separated by 1% (wt/vol) agarose gel electrophoresis, as described by Thornton et al.23 or 7.5% SDS-polyacrylamide gel electrophoresis (PAGE). The proteins were then transferred to nitrocellulose membranes (Millipore; Bedford, MA) by vacuum (agarose gels) or electroblot (SDS-PAGE) and incubated with the OC125 and H185 antibodies, as previously described.20 For lectin blot, membranes were incubated with 1% polyvinylpyrrolidone in 0.1% Tween-Tris-buffered saline (TTBS; pH 7.5), for 1 hour and then with biotin-labeled peanut agglutinin (PNA, 25 μg/mL) to the mucin-associated T-antigen epitope or Maackia amurensis agglutinin (MAA, 100 μg/mL) to terminal α(2–3) sialic acid for 90 minutes at room temperature on a shaker. Membranes were developed with an ABC kit (Vectastain; Vector Laboratories) followed by incubation chemiluminescence substrate (SuperSignal West Pico; Pierce).
Biotinylation of Cell Surface Proteins
HCLE cell surface proteins were biotinylated and isolated (Pinpoint Cell Surface Protein Isolation Kit; Pierce) according to the manufacturer’s instructions. Samples were analyzed by lectin blot as described earlier.
Static Adhesion Assay
Static adhesion assays were performed on HCLE cells and corneal fibroblasts grown in one-well slides in the presence or absence of benzyl-α-GalNAc, as described by Huang et al.24 Briefly, the cell culture slides were washed with HEPES buffer (0.05 M HEPES, 0.15 M NaCl, 1 mM CaCl2, 1 mM MgCl2; pH 7.4) and incubated with a suspension (1 × 106 cells/mL) of trypsinized HCLE cells labeled with 6-carboxyfluorescein diacetate (6-CFDA) or with fibroblasts labeled with carboxyfluorescein succinimidyl ester (CFSE), according to the manufacturer’s instructions (Invitrogen-Molecular Probes, Inc., Eugene, OR). The cells were incubated at room temperature for 1 hour without fixation. Nonadherent cells were aspirated and the cell culture slides were washed with six changes of PBS. The slides were observed under a fluorescence microscope (Eclipse E800; Nikon Instruments, Tokyo, Japan).
Dynamic Flow Assay
One-well slides with HCLE cells grown in the presence or absence of benzyl-α-GalNAc were fitted under vacuum to a parallel plate chamber (Glycotech, Gaithersburg, MD) using a 0.005-in. gasket according to the manufacturer’s instructions. 6-CFDA-labeled HCLE cells in serum-free media (5 × 105 cells/mL) were perfused at room temperature into the chamber using a syringe pump (Harvard Apparatus) at a flow rate of 0.15 mL/min (shear stress of 0.8 dyn/cm2) as previously described.24 HCLE cell–cell interactions were visualized in real time by video microscopy on an inverted-stage microscope equipped with both phase and epifluorescence optics (Eclipse TE2000-S; Nikon Instruments). Transient adhering (rolling) cells were visualized after 30 seconds of flow by digital video recordings using a 10× objective lens on eight fields of view per slide. The number of rolling cells in each field was manually determined by superimposing five consecutive frames of the video recordings, corresponding to a total time of 0.167 seconds. Rolling cells (cells that moved <200 μm in five frames) were counted and compared among the different conditions.
Cationized Ferritin Binding to HCLE Cells
Cationized ferritin (CF; Electron Microscopy Sciences, Hatfield, PA) was labeled with fluorescein isothiocyanate (FITC) by using an FITC labeling kit (Calbiochem, La Jolla, CA) according to the manufacturer’s instructions. The resultant molar FITC-to-CF ratios obtained varied between 1.2 and 2.8. CF-FITC binding to HCLE cells was determined by using a previously described protocol.25 Briefly, cells in culture were incubated for 1 hour at 4°C with 100 μg/mL of CF-FITC in 500 μL of culture medium. After washing twice with PBS, the cells were fixed in 4% paraformaldehyde for 30 minutes, washed with PBS, coverslipped in mounting medium (Vectashield; Vector Laboratories) plus propidium iodide, and photographed at 4× with a fluorescence microscope (Nikon). Images were automatically analyzed (CellProfiler cell image–analysis software; available at www.cellprofiler.org/open-source software provided by The Broad Institute, Massachusetts Institute of Technology, Cambridge, MA).
Results
Inhibition of Terminal Mucin O-Glycan Biosynthesis
Benzyl-α-GalNAc is a chemical primer commonly used to suppress the elaboration of cell surface O-glycans.17,22,26,27 Cells treated with this compound express truncated O-linked carbohydrates without affecting cell viability or doubling time.15,21,28
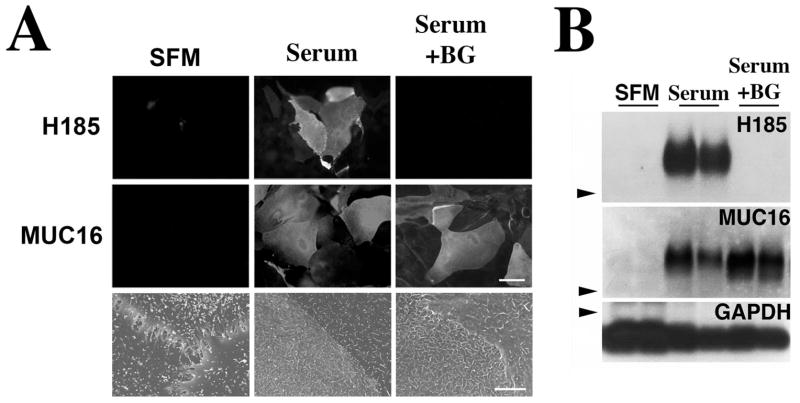
Inhibition of terminal mucin O-glycan biosynthesis was performed in a corneal epithelial cell culture system in which treatment of cells with serum for 7 days results in cellular stratification and expression of glycosylated, cell surface mucins.18 As shown in Figure 1A, the cell surface-associated mucin MUC16 and its terminal O-acetylated sialic acid epitope—designated H185—were present on the apical cell surface of islands of epithelial cells in the presence of serum (Serum), but not in cells grown in SFM. In the presence of benzyl-α-GalNAc (Serum+BG), MUC16, but not the mucin-associated carbohydrate epitope H185, was detected on the apical surface of HCLE cells. By Western blot, an increase in OC125 antibody binding to MUC16 was observed after benzyl-α-GalNAc treatment compared with the control (Fig. 1B), which could be explained by the susceptibility of this antibody to glycosylation of the mucin epitope.20 As determined by scanning electron microscopy, addition of serum to corneal epithelial cells resulted in the formation of numerous folds in the form of microplicae or microridges in the plasma membrane that were not altered by the addition of benzyl-α-GalNAc.
Figure 1.
Alteration of mucin O-glycosylation in HCLE cells treated with benzyl-α-GalNAc. Addition of serum to HCLE cell cultures triggered the biosynthesis of cell surface-associated MUC16 and its H185 carbohydrate epitope, as determined by immunofluorescence (A) and Western blot (B), as well as the appearance of microplicae at the cell surface, as determined by scanning electron microscopy. In the presence of benzyl-α-GalNAc, HCLE cells did not synthesize the MUC16-associated H185 carbohydrate epitope. The inhibitor did not affect either the biosynthesis of MUC16 or the cell surface ultrastructural features of HCLE cells compared with serum treatment alone. Arrowheads: position of the 250-kDa molecular weight marker in the agarose gel. GAPDH was used as a loading control. Scale bars: 60 μm (immunofluorescence images), 5 μm (scanning electron microscopy images).
To further evaluate the effect of benzyl-α-GalNAc in modifying cell surface carbohydrates, cell surface proteins on HCLE cells were biotinylated, purified, and analyzed by lectin blot (Fig. 2). In the presence of serum, MAA binding revealed several bands corresponding to glycoproteins with terminal α(2–3) sialic acid linkages. Treatment with benzyl-α-GalNAc decreased binding of MAA to high-molecular–weight glycoproteins, which correlates to a lack of O-acetyl sialic acid in the high-molecular-weight mucin MUC16, as shown by the H185 staining in Figure 1. The remaining MAA staining may represent sialic acid on nonmucin type O-glycans, which are not affected by benzyl-α-GalNAc. PNA binding to the mucin-associated T-antigen epitope was observed after benzyl-α-GalNAc treatment, probably as a consequence of the removal of terminal sialic acid on the mucin. Benzyl-α-GalNAc has been shown to increase the PNA binding to CD44 at the cell surface in B16BL6 melanoma cells by decreasing the sialylation of the sugar chains.27 These results suggest that benzyl-α-GalNAc alters mucin O-glycosylation in HCLE cells by affecting terminal carbohydrates (e.g., sialic acid) on O-glycan chains, but not core structures, such as those recognized by PNA.
Figure 2.
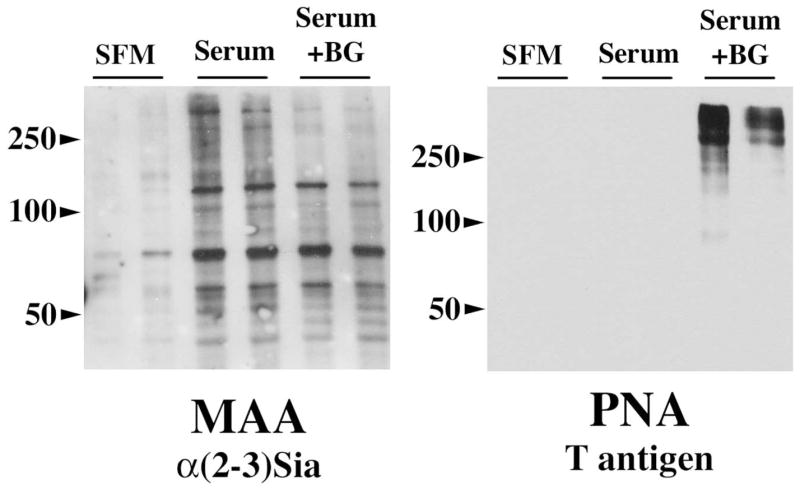
Effect of benzyl-α-GalNAc on biotinylated cell surface glycoproteins in HCLE cells. As determined by MAA binding, benzyl-α-GalNAc decreased O-linked sialylation on high molecular weight glycoproteins, which correlates with decreased binding of the H185 antibody to the cell surface–associated mucin MUC16 after benzyl-α-GalNAc treatment (Fig. 1). Binding of PNA to the mucin-associated T-antigen increased after benzyl-α-GalNAc treatment, most likely as a consequence of the reduced levels of terminal sialic acid on the cell surface mucins. Experiments were performed in duplicate.
Static Adhesion Assays
In these experiments, cells in suspension were labeled with a fluorescent dye and incubated with cultures of HCLE cells or human corneal fibroblasts (which do not express mucins) cultured with or without benzyl-α-GalNAc (Fig. 3). The adhesive character of the apical cell surface was measured by static adhesion assay as described in the Methods section.
Figure 3.
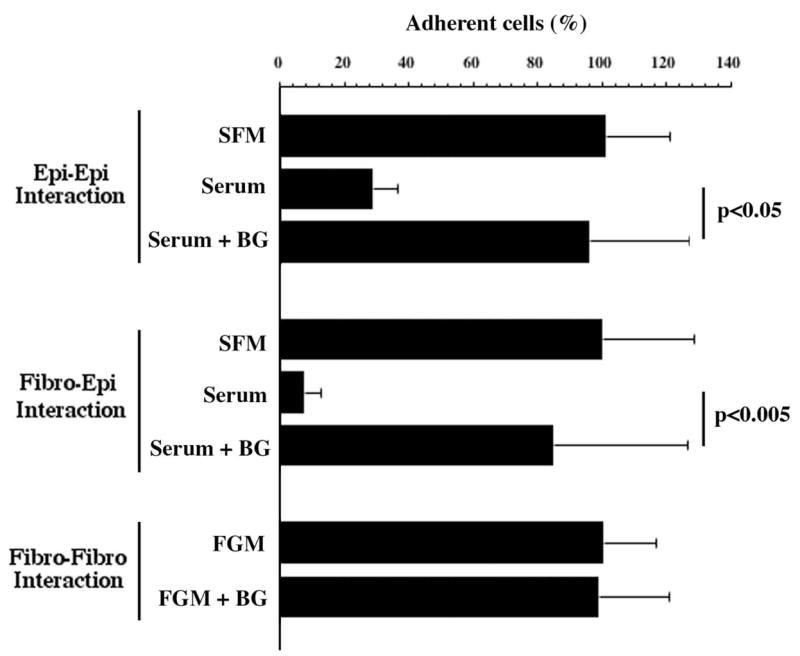
Mucin O-glycans contribute to the prevention of epithelial cell surface adhesion under static conditions. Fluorescein-labeled HCLE cells and human fibroblasts in suspension showed reduced binding to HCLE cells grown under conditions that promote mucin O-glycan biosynthesis (Serum), compared with serum-free media (SFM) or to cells treated with the inhibitor benzyl-α-GalNAc (Serum+BG). In control experiments, fluorescein-labeled fibroblasts showed similar levels of adhesion to fibroblasts grown in the presence (FGM+BG) or absence (FGM) of benzyl-α-GalNAc, indicating that benzyl-α-GalNAc influences cell adhesion through the inhibition of O-glycosylation in mucin-expressing cells. Error bars, SEM.
Induction of mucin and mucin O-glycan biosynthesis in HCLE cells after addition of serum (Serum) resulted in a decrease in corneal epithelial cell adhesion compared with SFM. When HCLE cells were treated with benzyl-α-GalNAc to inhibit terminal mucin O-glycan biosynthesis, there was a significant increase in cell adhesion, suggesting that an intact mucin O-glycan chain is necessary to maintain the antiadhesive character of the epithelial cell surface. Similar results were obtained when testing the adhesion of labeled fibroblasts to HCLE cells treated with benzyl-α-GalNAc. Treatment of the HCLE cell surface with neuraminidase from Arthrobacter ureafaciens under conditions that remove sialic acid and their O-acetylated derivatives in HCLE cells, as determined by immunofluorescence and HPLC,20 did not affect adhesion in the static assay (results not shown). Although it is possible that some sialic acids not affected by the sialidase treatment are still present on the cell surface, this result suggests that sialic acid alone is not directly involved in preventing cell–cell adhesion.
To determine whether benzyl-α-GalNAc influences cell adhesion by an O-glycosylation-independent mechanism, corneal fibroblasts, which do not synthesize mucins,29 were also treated with the inhibitor. As shown in Figure 3, the adhesive character of the cell surface of corneal fibroblasts treated with benzyl-α-GalNAc (FGM+BG) did not differ from control fibroblasts (FGM), indicating that benzyl-α-GalNAc influences cell adhesion through the inhibition of O-glycosylation in mucin-expressing cells.
Flow Adhesion Assay
The role of mucin O-glycans in preventing apical cell adhesion was also evaluated using dynamic flow assays under defined shear stress. In these experiments, transient adherent cells were defined as cells moving less than 200 μm in five consecutive frames (equivalent to 0.167 seconds) in the video recordings (representative movies available online at http://www.iovs.org/cgi/content/full/49/1/197/DC1). As shown in Figure 4, fluorescein-labeled HCLE cells transiently adhered to cultures of undifferentiated cells (SFM, Movie 1). In cell cultures producing O-glycans (Serum, Movie 2), cells rolled faster than in the SFM condition, as evidenced by the presence of smears (Fig. 4B, long arrows) in the superimposed images. In the presence of benzyl-α-GalNAc (Serum+BG, Movie 3), cells transiently adhered to the apical surface of HCLE cells, forming a pattern similar to cultures grown in SFM. During the course of the experiments, no resting adherent cells were detected under the shear stress of 0.8 dyn/cm2 used in the assay. Decreased shear stress resulted in similar rolling patterns among the three experimental conditions used in this assay.
Figure 4.
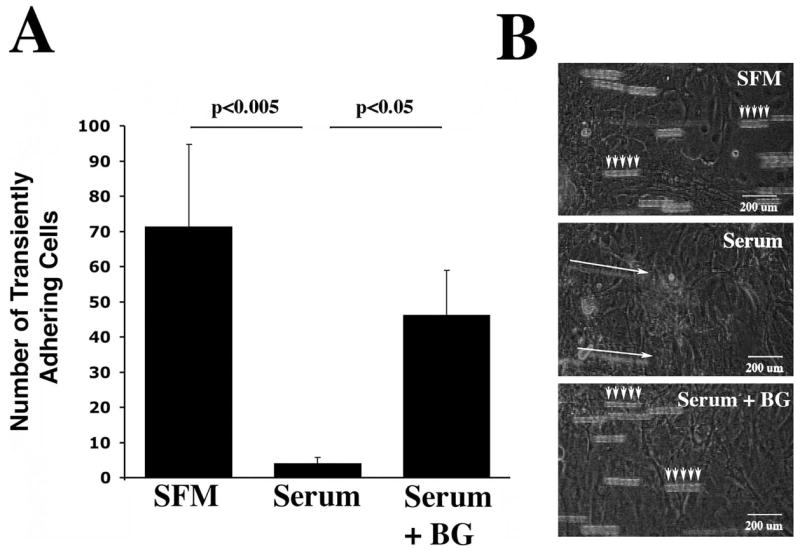
Quantification of the number of transient adhering (rolling) cells under dynamic flow conditions in HCLE cells cultured under different conditions. (A) Inhibition of O-glycosylation with benzyl-α-GalNAc (Serum+BG) increased the number of rolling cells compared with the serum condition (Serum). Rolling cells were defined as cells moving less than 200 μm in five superimposed frames (t = 0.167 s). Error bars, SEM. (B) Representative images of different experimental growth conditions obtained after superimposing five consecutive frames taken from one field of view. In these experiments, the bright-field channel was open to allow visualization of HCLE cells grown on the culture slide. Movies corresponding to images are available online at http://www.iovs.org/cgi/content/full/49/1/197/DC1 (SFM, Movie 1; Serum, Movie 2; Serum+BG, Movie 3).
Cationized Ferritin Binding to HCLE Cells
Previous data have shown that negative charges on cell surface glycoproteins may play a key role in preventing cellular adhesion due to negative charge repulsion.30 To test this hypothesis in our experimental model, we incubated HCLE cells with CF-FITC, a molecular probe that has been used to detect negative residues on plasma membranes. As shown in Figure 5, cells grown in SFM, which promotes apical adhesion, have an overall, homogeneous distribution of negative charges on the plasma membrane. Induction of mucin O-glycosylation by serum results in the appearance of apical islands lacking negative charges. These islands have been shown to contain the cell surface mucin MUC16,31 suggesting that cell surface–associated mucins and their O-glycans lack negative charges in HCLE cells. Incubation of HCLE cells with benzyl-α-GalNAc resulted in increased binding of CF-FITC to the cell surface, indicating that negative charges are not involved in the antiadhesive properties of mucin O-glycans, since the greater number of negative charges correlates with increased adhesion under static and dynamic flow conditions (Figs. 3, 4).
Figure 5.
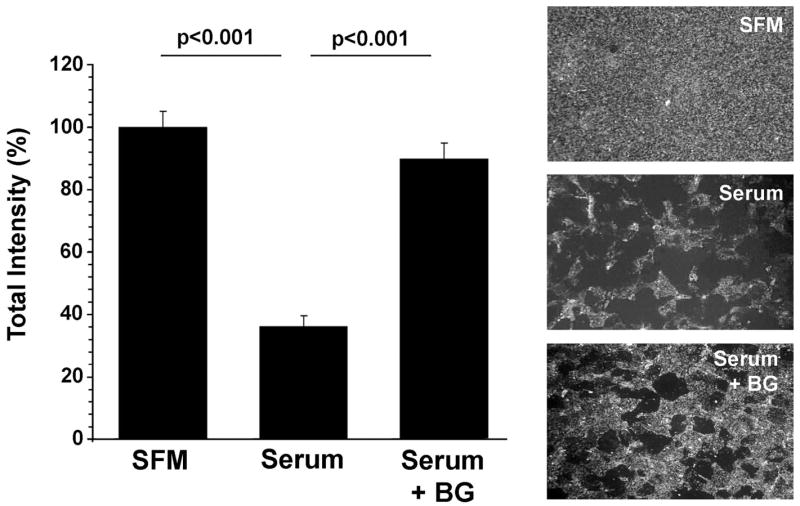
Cationized ferritin binding to HCLE cells after benzyl-α-GalNAc treatment. Apical surfaces of undifferentiated cells (SFM) and differentiated cells grown in the presence of benzyl-α-GalNAc (Serum+BG) have more negative charges than cells grown in the presence of serum (Serum). These results suggest that negative charges do not play a role in preventing cellular adhesion in HCLE cells. Error bars, SEM.
Discussion
The role of mucin-type O-glycans on the cell surface glycocalyx of normal, wet-surfaced epithelia is poorly understood. We performed in vitro assays to examine whether apical, cell surface-associated O-glycans contribute to the modulation of adhesion on corneal epithelial surfaces. Our results using static and dynamic flow adhesion assays showed that the character of the cell surface of corneal epithelial cells producing O-glycans was more antiadhesive than that of cells with truncated mucin O-glycans and that negative charges on the cell surface were not involved in preventing adhesion. These data suggest that, under normal conditions, mucin-type O-glycans—which protrude high above the plasma membrane—play a role in preventing apical epithelial surface adhesion and may contribute to the boundary lubrication of opposite membranes in mucosal tissues.
The length of cell surface–associated mucins has been estimated to extend at least 200 to 500 nm above the cell membrane, far above other glycoproteins in the glycocalyx.11,14,32 It is generally recognized that this extended mucin protein backbone on cell surface–associated mucins confers an antiadhesive character to cell surfaces. Transfecting both normal and tumor epithelial cells with MUC1 constructs containing increasing numbers of tandem repeats yields a reduction in the number of cell–cell interactions, indicating that the antiadhesive effect depends on the size and level of expression of the mucin.12–14 The antiadhesive character of mucins has also been observed by atomic-force microscopy on mucins from human conjunctival tissue, where the number of mucin–mucin interactions is minimal compared with mucin–mica interactions.33 O-linked carbohydrates are important in maintaining the highly extended and rigid structure of the mucin protein backbone. Clustered O-glycans induce the peptide core in mucin-type glycoproteins to adopt a stiff and extended conformation that prevents folding into a globular structure.34 A study using MUC1 synthetic peptides with conformational and dynamic analyses showed that glycosylation at the central threonine within the PDTRP core epitope region shifts the conformational equilibrium away from a type I β-turn conformation toward a more rigid and extended state.35 Consequently, densely packed O-glycan chains on cell surface–associated mucins have been postulated to induce the extended rodlike structure in native tissues. Our experiments showing increased cell surface adhesion after truncation of O-glycan chain elongation with benzyl-α-GalNAc are consistent with the concept that O-glycans contribute to the maintenance of an extended structure of the cell surface–associated mucin that prevents cell adhesion. After blocking the synthesis of the hydrophilic carbohydrate side chains, the extended structure of the membrane-associated mucins may collapse36 and/or expose a more adhesive, hydrophobic protein backbone that facilitates cellular attachment.37
In addition to their role in maintaining an extended structure, mucin O-glycans contain negatively charged sialic acids that may also prevent cell adhesion by giving glycoproteins a strong negative charge that would favor charge repulsion.10,11 Glycoproteins with abundant levels of sialic acid, such as podocalyxin—a major membrane protein of the glomerular epithelium—or the polysialylated form of the neural cell adhesion molecule (N-CAM), have an antiadhesive function in the glycocalyx that is sialidase dependent.30,38 Removal of sialic acid by neuraminidase in cells transfected with the cell surface–associated mucin MUC1 has, however, yielded controversial results in aggregation assays—showing that sialic acid residues are either not required for the antiadhesive effect of the mucin13 or partially contribute to the modulation of cell adhesion.12 In these experiments, the length of the mucin molecule seemed to determine the role of sialic acid. Consequently, Wesseling et al.13 have proposed that sialic acid mediates cell adhesion by charge repulsion in mucin molecules containing a low number of tandem repeats (as shown in MUC1 transfectants containing eight repeats), whereas in a longer MUC1, the extended state of the mucin—and not charge repulsion—becomes the predominant factor in modulating cell adhesion. Our observations using native (full-length) mucin produced by human corneal epithelial cells are in line with this hypothesis—truncation of mucin O-glycans with benzyl-α-GalNAc treatment increased cell adhesion independent of sialidase treatment, suggesting that benzyl-α-GalNAc may compromise the extended structure of the native mucin and that sialic acid does not play a role in modulating adhesion in the surface-associated mucin. Increased adhesion after benzyl-α-GalNAc treatment that is independent of sialidase treatment has also been observed in hepatocarcinoma cells transfected with the carcinoma-associated glycoprotein dysadherin.39 Determining the exact extent to which benzyl-α-GalNAc affects the elongation of O-glycans would be helpful in identifying carbohydrate structures that might be necessary to maintain the antiadhesive properties of the mucin. In our experiments, charge repulsion did not appear to contribute to the antiadhesive character of the cell surface, since the amount of negative charges increased after treatment with benzyl-α-GalNAc, perhaps by exposing negative residues on the plasma membrane that would otherwise have been hindered by the extended structure of the fully glycosylated mucin.
Cell surface–associated mucins and their O-glycans are constitutively expressed on apical membranes of epithelia that require a lumen and are, thus, antiadhesive. In mucosae that border the external environment, such as the ocular surface, oral cavity, and reproductive tract, dryness is a common and frequently chronic disorder characterized by adhesiveness of the apical surface. It is possible to speculate that altered biosynthesis of O-glycans in these tissues contributes to adhesion and lack of boundary lubrication. Altered mucin-type O-glycosylation has been demonstrated in drying diseases. In patients with dry eye, this altered state is evidenced in the lack of binding of the monoclonal antibody H185, which recognizes O-acetyl sialic acid on MUC16, to apical cell membranes on stratified squamous conjunctival epithelium.7 Using antibodies specific to polypeptide GalNAc-transferases, a family of enzymes that initiate mucin-type O-glycosylation, we have also shown lack of binding to dry keratinized areas of the ocular surface in patients with ocular cicatricial pemphigoid.6 Studies using a panel of lectins have shown that the degree of glycosylation is altered in the labial salivary glands of patients with Sjögren’s syndrome—a drying disease that affects both ocular and oral mucosae.8,9 Although the specific structural changes in the mucin O-glycan chain in these patients have not yet been defined, the results suggest that alteration in mucin O-glycosylation may compromise the maintenance of a hydrated and antiadhesive epithelial surface. Conducting experiments on the role of mucin O-glycans in a dry chamber setting would also provide information and parameters that would more closely mimic clinical drying conditions.
In summary, our results in static and dynamic flow experimental conditions indicate that mucin O-glycans on corneal epithelial cells contribute to the prevention of apical cell surface adhesion and that this phenomenon is not mediated by charge repulsion. Alteration in the distribution of mucins and their O-glycosylation in drying diseases, such as dry eye, may result in adherence and damage to the epithelial surface by abrasive stress.
Supplementary Material
Acknowledgments
Supported by National Eye Institute Grants R01EY014847 (PA) and R01EY03306 (IKG).
The authors thank James Zieske, Schepens Eye Research Institute, for providing human corneal fibroblasts.
Footnotes
Disclosure: M. Sumiyoshi, None; J. Ricciuto, None; A. Tisdale, None; I.K. Gipson, None; F. Mantelli, None; P. Argüeso, None
References
- 1.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 2.Argueso P, Gipson IK. Epithelial mucins of the ocular surface: structure, biosynthesis and function. Exp Eye Res. 2001;73:281–289. doi: 10.1006/exer.2001.1045. [DOI] [PubMed] [Google Scholar]
- 3.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Gipson IK, Hori Y, Argueso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004;2:131–148. doi: 10.1016/s1542-0124(12)70149-0. [DOI] [PubMed] [Google Scholar]
- 5.Gendler SJ, Spicer AP. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- 6.Argueso P, Tisdale A, Mandel U, Letko E, Foster CS, Gipson IK. The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Invest Ophthalmol Vis Sci. 2003;44:86–92. doi: 10.1167/iovs.02-0181. [DOI] [PubMed] [Google Scholar]
- 7.Danjo Y, Watanabe H, Tisdale AS, et al. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–2609. [PubMed] [Google Scholar]
- 8.Penaloza A, Decaestecker C, Ribai P, et al. Sialic acid residues in the labial salivary glands from Sjogren’s syndrome patients. Clin Exp Rheumatol. 1999;17:713–717. [PubMed] [Google Scholar]
- 9.Sidagis J, Ueno K, Wang ZH, Hanamure Y, Furuta S, Ohyama M. Expression of glycoconjugates in normal and Sjogren’s syndrome labial glands. Acta Otolaryngol. 1997;117:871–877. doi: 10.3109/00016489709114217. [DOI] [PubMed] [Google Scholar]
- 10.Patton S, Gendler SJ, Spicer AP. The epithelial mucin, MUC1, of milk, mammary gland and other tissues. Biochim Biophys Acta. 1995;1241:407–423. doi: 10.1016/0304-4157(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 11.Hilkens J, Ligtenberg MJ, Vos HL, Litvinov SV. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem Sci. 1992;17:359–363. doi: 10.1016/0968-0004(92)90315-z. [DOI] [PubMed] [Google Scholar]
- 12.Ligtenberg MJ, Buijs F, Vos HL, Hilkens J. Suppression of cellular aggregation by high levels of episialin. Cancer Res. 1992;52:2318–2324. [PubMed] [Google Scholar]
- 13.Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol Biol Cell. 1996;7:565–577. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komatsu M, Carraway CA, Fregien NL, Carraway KL. Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J Biol Chem. 1997;272:33245–33254. doi: 10.1074/jbc.272.52.33245. [DOI] [PubMed] [Google Scholar]
- 15.Kuan SF, Byrd JC, Basbaum C, Kim YS. Inhibition of mucin glycosylation by aryl-N-acetyl-alpha-galactosaminides in human colon cancer cells. J Biol Chem. 1989;264:19271–19277. [PubMed] [Google Scholar]
- 16.Bertozzi CR, Kiessling LL. Chemical glycobiology. Science. 2001;291:2357–2364. doi: 10.1126/science.1059820. [DOI] [PubMed] [Google Scholar]
- 17.Prescher JA, Bertozzi CR. Chemical technologies for probing glycans. Cell. 2006;126:851–854. doi: 10.1016/j.cell.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Gipson IK, Spurr-Michaud S, Argueso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal-limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–2506. doi: 10.1167/iovs.02-0851. [DOI] [PubMed] [Google Scholar]
- 19.Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK. MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci. 2003;44:2487–2495. doi: 10.1167/iovs.02-0862. [DOI] [PubMed] [Google Scholar]
- 20.Argueso P, Sumiyoshi M. Characterization of a carbohydrate epitope defined by the monoclonal antibody H185: sialic acid O-acetylation on epithelial cell-surface mucins. Glycobiology. 2006;16:1219–1228. doi: 10.1093/glycob/cwl041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Byrd JC, Yoon WH, Kim YS. Effect of benzyl-alpha-GalNAc, an inhibitor of mucin glycosylation, on cancer-associated antigens in human colon cancer cells. Oncol Res. 1992;4:507–515. [PubMed] [Google Scholar]
- 22.Ulloa F, Franci C, Real FX. GalNAc-alpha-O-benzyl inhibits sialylation of de novo synthesized apical but not basolateral sialoglycoproteins and blocks lysosomal enzyme processing in a post-trans-Golgi network compartment. J Biol Chem. 2000;275:18785–18793. doi: 10.1074/jbc.M000510200. [DOI] [PubMed] [Google Scholar]
- 23.Thornton DJ, Khan N, Sheehan JK. Separation and identification of mucins and their glycoforms. Methods Mol Biol. 2000;125:77–85. doi: 10.1385/1-59259-048-9:077. [DOI] [PubMed] [Google Scholar]
- 24.Huang MC, Laskowska A, Vestweber D, Wild MK. The alpha (1,3)-fucosyltransferase Fuc-TIV, but not Fuc-TVII, generates sialyl Lewis X-like epitopes preferentially on glycolipids. J Biol Chem. 2002;277:47786–47795. doi: 10.1074/jbc.M208283200. [DOI] [PubMed] [Google Scholar]
- 25.Matsui H, Johnson LG, Randell SH, Boucher RC. Loss of binding and entry of liposome-DNA complexes decreases transfection efficiency in differentiated airway epithelial cells. J Biol Chem. 1997;272:1117–1126. doi: 10.1074/jbc.272.2.1117. [DOI] [PubMed] [Google Scholar]
- 26.Dube DH, Prescher JA, Quang CN, Bertozzi CR. Probing mucin-type O-linked glycosylation in living animals. Proc Natl Acad Sci USA. 2006;103:4819–4824. doi: 10.1073/pnas.0506855103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano T, Matsui T, Ota T. Benzyl-alpha-GalNAc inhibits sialylation of O-glycosidic sugar chains on CD44 and enhances experimental metastatic capacity in B16BL6 melanoma cells. Anticancer Res. 1996;16:3577–3584. [PubMed] [Google Scholar]
- 28.Huet G, Hennebicq-Reig S, de Bolos C, et al. GalNAc-alpha-O-benzyl inhibits NeuAcalpha2–3 glycosylation and blocks the intracellular transport of apical glycoproteins and mucus in differentiated HT-29 cells. J Cell Biol. 1998;141:1311–1322. doi: 10.1083/jcb.141.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inatomi T, Spurr-Michaud S, Tisdale AS, Zhan Q, Feldman ST, Gipson IK. Expression of secretory mucin genes by human conjunctival epithelia. Invest Ophthalmol Vis Sci. 1996;37:1684–1692. [PubMed] [Google Scholar]
- 30.Takeda T, Go WY, Orlando RA, Farquhar MG. Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11:3219–3232. doi: 10.1091/mbc.11.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Argueso P, Tisdale A, Spurr-Michaud S, Sumiyoshi M, Gipson IK. Mucin characteristics of human corneal-limbal epithelial cells that exclude the rose bengal anionic dye. Invest Ophthalmol Vis Sci. 2006;47:113–119. doi: 10.1167/iovs.05-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bramwell ME, Wiseman G, Shotton DM. Electron-microscopic studies of the CA antigen, epitectin. J Cell Sci. 1986;86:249–261. doi: 10.1242/jcs.86.1.249. [DOI] [PubMed] [Google Scholar]
- 33.Berry M, McMaster TJ, Corfield AP, Miles MJ. Exploring the molecular adhesion of ocular mucins. Biomacromolecules. 2001;2:498–503. doi: 10.1021/bm000145y. [DOI] [PubMed] [Google Scholar]
- 34.Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990;15:291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- 35.Schuman J, Campbell AP, Koganty RR, Longenecker BM. Probing the conformational and dynamical effects of O-glycosylation within the immunodominant region of a MUC1 peptide tumor antigen. J Pept Res. 2003;61:91–108. doi: 10.1034/j.1399-3011.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 36.Shogren R, Gerken TA, Jentoft N. Role of glycosylation on the conformation and chain dimensions of O-linked glycoproteins: light-scattering studies of ovine submaxillary mucin. Biochemistry. 1989;28:5525–5536. doi: 10.1021/bi00439a029. [DOI] [PubMed] [Google Scholar]
- 37.Ciborowski P, Finn OJ. Non-glycosylated tandem repeats of MUC1 facilitate attachment of breast tumor cells to normal human lung tissue and immobilized extracellular matrix proteins (ECM) in vitro: potential role in metastasis. Clin Exp Metastasis. 2002;19:339–345. doi: 10.1023/a:1015590515957. [DOI] [PubMed] [Google Scholar]
- 38.Yang P, Yin X, Rutishauser U. Intercellular space is affected by the polysialic acid content of NCAM. J Cell Biol. 1992;116:1487–1496. doi: 10.1083/jcb.116.6.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuiji H, Takasaki S, Sakamoto M, Irimura T, Hirohashi S. Aberrant O-glycosylation inhibits stable expression of dysadherin, a carcinoma-associated antigen, and facilitates cell-cell adhesion. Glycobiology. 2003;13:521–527. doi: 10.1093/glycob/cwg065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.