Abstract
Endogenous retroviral gene products have been found in some human tumors, and therefore, may serve as antigens for immunotherapy approaches. The murine colorectal carcinoma CT26 and melanoma B16 have recently been found to express the endogenous retroviral gene products gp70 and p15E, respectively, that can serve as antigens recognized by T cells. To date, though, there has been no demonstration of tumor treatment using an endogenous retroviral protein. In this study, we demonstrate that mice immunized with recombinant vaccinia encoding the gp70 H2-Ld-restricted minimal determinant were protected from CT26 tumor challenge. Splenocytes from mice immunized with vaccinia gp70 specifically secreted IFN-γ in response to gp70 peptide-pulsed stimulators. Although this strategy could protect against subsequent tumor challenge, it was ineffective against established tumors. Therefore, to investigate the treatment of established CT26 or B16 lung metastases, mice were treated with cultured dendritic cells (DCs) pulsed with gp70 or p15E peptide. Significant inhibition of established lung metastases required immunization with peptide-pulsed DCs pretreated with CD40 ligand that has been demonstrated to increase the T-cell stimulatory activity of DCs. The ability to immunize against endogenous retroviral tumor antigens may have relevance in the induction of antitumor immunity for some human cancers.
INTRODUCTION
Many endogenous retroviral elements have been identified in humans. These elements are thought to have arisen from rare infection and integration events of retrovirus into germ-line cells early in evolution. The sequences generally encode defective proviruses, although transcripts and protein products of portions of the provirus have been detected in a number of tissues and tumor cell lines. Their relevance as TAAs4 is under investigation. HERV gag gene products have been found in the sera of patients with human seminoma (1) and renal cell carcinoma (2). In addition, expression of HERV-derived pol has been demonstrated in breast (3) and colon cancers (4). Transcripts of HERV-R and HERV-H have been found in normal and neoplastic tissues (5, 6). RNA of HERV-K has been detected in many tissues (7), and antibodies to HERV-K gag and env proteins have been reported in human sera (8). The role of HERV sequences in cellular transformation to a malignant phenotype is unclear, although high expression of retroelements increases the risk of genetic mutation attributable to transposition events (9). In addition, expression levels of HERV can be correlated with disease in some human malignancies (8). Therefore, HERV-derived proteins may make suitable targets for tumor vaccines. Indeed, endogenous retroviral protein antigens recognized by T cells have been detected in human melanomas.5
Endogenous retroviral gene products can serve as TAAs in some murine tumors. The env products of endogenous MuLV such as gp70, have been found to be expressed in CT26 colon carcinoma of BALB/c mice (10), whereas they are absent in normal BALB/c mouse tissues. A number of other tumors including B16 melanoma (11, 12) has also been demonstrated to express gp70. In addition, the gp70 protein has been found to be an antigen for CD4+ T cells in murine Friend leukemia (13), in CT26 (14), and for CD8+ cells specific for the CT26 tumor (10). Adoptively transferred T cells specific for gp70 can treat mice bearing the CT26 tumor, and mice immunized with a modified peptide epitope of gp70 could be protected from subsequent tumor challenge (15). However, active immunization with gp70 resulting in treatment of an established tumor has never been demonstrated.
CTLs specific for the transmembrane component of the retroviral env protein p15E have also been found in tumor-bearing mice (16). In addition, p15E has been demonstrated to be a MHC class I restricted T-cell antigen expressed in the MC38 and B16 tumor cell lines derived from C57BL/6 mice (17). Tumor-infiltrating lymphocyte cultures obtained from a methylcholanthrene-induced fibrosarcoma and MC-38 were shown to recognize p15E in a H-2Kb-restricted fashion. Furthermore, p15E-specific CTL lines generated from splenocyte cultures by in vitro peptide stimulation were reactive against several p15E-expressing tumor lines. However, there has been no demonstration of active immunization against p15E to date.
A variety of immunization strategies have been explored through animal models with the ultimate goal of discovering reliable methods for actively immunizing cancer patients against their tumors (18). Importantly the majority of these models demonstrate antitumor activity against chemically induced tumors or tumors expressing model antigens and, despite the identification of several antigen targets, there are few examples in the literature of active immunization against truly native antigens. Presumably, the repertoire of T cells recognizing native antigens is diminished by central and/or peripheral tolerance. According to this view, efforts to immunize using self-antigens would be additionally confounded by the fact that the remaining autoreactive T cells would have only low to intermediate affinity for their respective antigens. Regardless of the mechanisms involved, actively immunizing against endogenous, self-derived antigens remains a difficult task indeed. The tumor-associated expression of endogenous retroviral proteins may make these proteins better candidates with which to generate an antitumor immune response. Furthermore, the association of endogenous retroviral protein expression with tumors of a range of histologies may enable these proteins to be used as immunogens against a variety of malignancies.
Among the numerous approaches to the development of cancer vaccines is the use of peptides, recombinant viral vaccines, and DC-based strategies (18). Because recombinant vaccinia can be effective in some murine tumor models, we initially evaluated the ability of vaccinia encoding the gp70 immunodominant epitope to immunize against the CT26 tumor. Although immunization with recombinant vaccinia could protect against subsequent tumor challenge, treatment of established tumor was not achieved. Therefore, we investigated the effect of immunization with in-vitro generated, bone marrow-derived, peptide-pulsed DCs on established tumor growth. DCs are central to antigen presentation and the initiation of an immune response. In addition, to enhance the immunogenic capacity of DCs against an established tumor we treated DCs with CD40 ligand (19), because interaction of CD40 ligand with CD40 expressed on DCs has been demonstrated to induce IL-12 production and increase T-cell stimulatory activity of DCs (20).
MATERIALS AND METHODS
Cell Lines
CT26 is a carcinogen-induced, undifferentiated colon carcinoma of BALB/c mice. CT26.CL25 is a β-gal-expressing cell line derived from CT26 by transduction with a retroviral vector encoding the lacZ gene driven by the Moloney MuLV long terminal repeat (21). B16 is a spontaneously arising melanoma of C57BL/6 mice propagated by Dr. Isaiah J. Fidler (M.D. Anderson Cancer Center, Houston, TX). NIH/3T3 is a mouse fibroblast cell line. 3T3-CD40L cells were generated by transducing NIH/3T3 cells with mouse CD40L cDNA. cDNA for mouse CD40L was isolated by reverse transcription-PCR from anti-CD3-activated mouse splenocytes and cloned into NotI/XhoI sites in the retroviral expression vector pSAMEN (22). The ecotropic producer cell line GP+E86 expressing CD40L was generated similarly as described previously (23), and supernatant was used to transduce NIH/3T3 followed by selection in G418. CD40L expression on NIH/3T3 was confirmed by flow cytometry. Cell lines were maintained at 37°C/5% CO2 in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 2 mm glutamine, 100 units/ml penicillin, 100 µg/ml streptomycin (all from Biofluids, Rockville, MD), and 1.25 µg/ml Amphotericin B (Fungizone; Life Technologies, Inc., Grand Island, NY).
Peptides
The gp70 peptide used in this study is a 9 amino acid sequence (SPSYVYHQF) derived from the endogenous MuLV envelope protein gp70 (10). Control H2-Ld-restricted peptide was β-gal (TPHPARIGL; Ref. 24). The H2-Kb-restricted peptide sequences used were derived from β-gal (DAPIYTNV) and p15E (KSPWFTTL). Peptides used to pulse DCs contained a 9 amino acid domain from the Tat regulatory protein from HIV, RKKRRQRRR, linked to the NH2 terminus of either β-gal or gp70. We have found Tat-linked peptides to be more effectively pulsed onto DCs.6
Vaccinia Vector Immunization
Recombinant vaccinia vectors were generated by insertion of either the gp70 or β-gal peptide sequence into the viral thymidine kinase coding region as described previously (25). Each peptide sequence in vaccinia was preceded by the ES signal peptide sequence (MRYMILGLLALAAVCSA) from the adenoviral E3/19K protein (26). BALB/c mice (5 per group) were immunized with either HBSS (Biofluids, Frederick, MD), the vaccinia gp70, or vaccinia β-gal (2 × 107 units in 0.5 ml) via the tail vein. Three weeks later, the mice were challenged i.v. with CT26 (1 × 106 cells in 0.5 ml of HBSS). Twelve days later the lungs were harvested, and lung metastases were enumerated by visualization after treatment with India ink as described previously (27). Statistical comparison between each treatment group in this and all of the other experiments was performed using the Kruskal-Wallis nonparametric test.
IFN-γ Production Assay
Four weeks after immunization, splenocytes were harvested from mice that had not been challenged with tumor. The splenocytes were restimulated with gp70 peptide in vitro (1 µg/ml) on the first day of culture. The culture was maintained for 6 days, and IL-2 was added on day 3 of culture. On day 6 of culture, in vitro stimulated splenocytes (1 × 105) were incubated in 96-well plates with 1 × 105 of either freshly harvested naive splenocytes or splenocytes pulsed with gp70, P1A, or β-gal peptide (1 µg/ml). Supernatant was harvested after overnight incubation and assayed for murine IFN-γ content by ELISA (Endogen) according to the manufacturer’s instructions.
DC Production and Tumor Treatment
Mouse DCs were derived from bone marrow using murine IL-4 (mIL-4) and granulocyte macrophage colony-stimulating factor (mGM-CSF) as described previously (27). Briefly, after in vitro depletion of CD4, CD8, B220, and MHC class II-positive cells, bone marrow cells (1 × 106/ml) were cultured in six-well plates in RPMI 1640 containing 20 mouse granulocyte macrophage-colony stimulating factor (ng/ml) mGM-CSF and 100 ng/ml mouse IL-4 (both from Biological Resources Branch, National Cancer Institute, Frederick, MD) with cytokine renewal every 2 days. After 6 days, nonadherent cells were replated onto 10-cm tissue culture plates, whereas adherent cells were discarded. Twenty-four h later nonadherent cells were harvested and pulsed for 4 h at 25°C with peptide in Opti-MEM medium (Life Technologies, Inc.) containing 1 µm of human β2-microglobulin, washed once, and used for mouse injections (5 × 105 i.v./mouse). To treat DCs with CD40L, day-6 cultured DCs were plated for 24 h onto irradiated 3T3 cells that had been transduced with a retroviral vector (SAMEN; Ref. 22) containing the cDNA sequence for cell-surface mouse CD40L. The control for CD40L treatment involved plating DCs onto 3T3 cells transduced with SAMEN vector alone. To determine the effect of peptide-pulsed DCs on established lung metastases, mice were injected with 1 × 106 CT26 i.v. followed 3 days later by 5 × 105 peptide-pulsed DCs injected i.v. Twelve days later lungs were removed and metastases enumerated.
RESULTS
Immunization with Vaccinia gp70 Induces gp70-specific Splenocytes
Splenocytes from mice immunized with recombinant vaccinia gp70 were assessed for their reactivity to gp70-pulsed targets. Four weeks after immunization, splenocytes were cultured for 6 days with gp70 peptide then cocultured with peptide-pulsed targets. T cells generated from vaccinia gp70-immunized mice produced high amounts of IFN-γ in response to incubation with gp70 peptide-pulsed irradiated splenocytes but not to P1A or β-gal-pulsed targets (Table 1). The requirement for incorporation of the gp70 peptide epitope into the vaccinia vector was evident from the observation that splenocytes from mice immunized with vaccinia β-gal secreted lower levels of IFN-γ after incubation with gp70-pulsed stimulators.
Table 1. Immunization with vaccinia gp70 generates gp70 peptide-reactive splenocytes.
Splenocytes were harvested from mice 4 weeks after immunization with either vaccinia gp70 or vaccinia β-gal and restimulated in vitro with gp70 peptide. After 6 days splenocytes were assessed for IFN-γ secretion (pg/ml) in response to the targets listed. Splenocytes from mice immunized with vaccinia gp70 secreted high amounts of IFN-γ specifically in response to incubation with gp70 peptide pulsed splenocytes.
| Targets |
|||||
|---|---|---|---|---|---|
| Immunogen | None | Fresh splenocytes | gp70-pulsed splenocytes | P1A-pulsed splenocytes | β-gal-pulsed splenocytes |
| Vaccinia β-gal | 1343 | 2088 | 2708 | 1978 | 3555 |
| Vaccinia gp70 | 1208 | 1536 | 20,543 | 1048 | 9526 |
Recombinant Vaccinia Virus gp70 Protects against CT26 Tumor
Recombinant vaccinia gp70 was next assessed for its ability to induce antitumor effects in vivo. Mice were immunized with HBSS or recombinant vaccinia vector encoding either gp70 or β-gal peptide and challenged 3 weeks later with either CT26 or CT26.CL25 (β-gal-transduced CT26) tumor i.v. Mice immunized with vaccinia gp70 were almost totally protected from lung metastasis formation (Table 2) after challenge with CT26. Protection against the CT26 tumor required the presence of gp70 peptide sequence in the vaccinia vector, because mice immunized with vaccinia vector containing β-gal peptide sequence developed significantly higher numbers of lung metastases (P2 < 0.05). Because CT26.CL25 also expresses gp70, we evaluated the effect of immunization with vaccinia gp70 on CT26.CL25 growth. Significant reduction in CT26.CL25 lung metastases resulted from immunization with vaccinia gp70. Mice were also protected from CT26.CL25 challenge but not CT26 challenge after immunization with vaccinia β-gal, thereby demonstrating the immunizing ability of vaccinia β-gal against β-gal-expressing tumor.
Table 2. Immunization with recombinant vaccinia gp70 protects against CT26 tumor challenge.
Mice were immunized with either vaccinia gp70, vaccinia β-gal, or HBSS and challenged i.v. 3 weeks later with CT26 or CT26.CL25 (β-gal-transduced CT26) tumor. Lung metastases were counted 12 days later and the mean value calculated (SD in parentheses). CT26-challenged mice that received vaccinia gp70 developed significantly fewer metastases than vaccinia β-gal- or HBSS-treated mice. Both vaccinia β-gal and vaccinia gp70 were effective in preventing tumor development in mice challenged with CT26.CL25.
DCs Pulsed with gp70 Peptide Can Treat Established Lung Metastases
Although immunization with recombinant vaccinia could protect against CT26, these methods were ineffective against established tumors (not shown). Therefore, we used peptide-pulsed cultured DCs in an attempt to treat established pulmonary metastases. In addition, because DC function is highly dependent on the level of activation (28), we also studied the use of CD40L-activated DCs in the treatment of established tumor. Mice were injected with 1 × 106 CT26 tumor cells i.v. followed 3 days later by peptide-pulsed DCs. DCs were incubated 24 h before peptide pulsing in the presence or absence of CD40L. Mice that received gp70-pulsed DCs had significantly less lung metastases than mice receiving either β-gal-pulsed DCs or HBSS alone (P2 < 0.01; Fig. 1), thereby confirming the requirement for gp70 to achieve therapeutic effect. An additional significant reduction in lung metastases over that achieved by gp70-pulsed DCs was observed by preincubation of DCs with CD40L (P2 < 0.01). β-Gal-pulsed DCs failed to reduce lung metastases regardless of whether DCs were preincubated with CD40L or not.
Fig. 1.
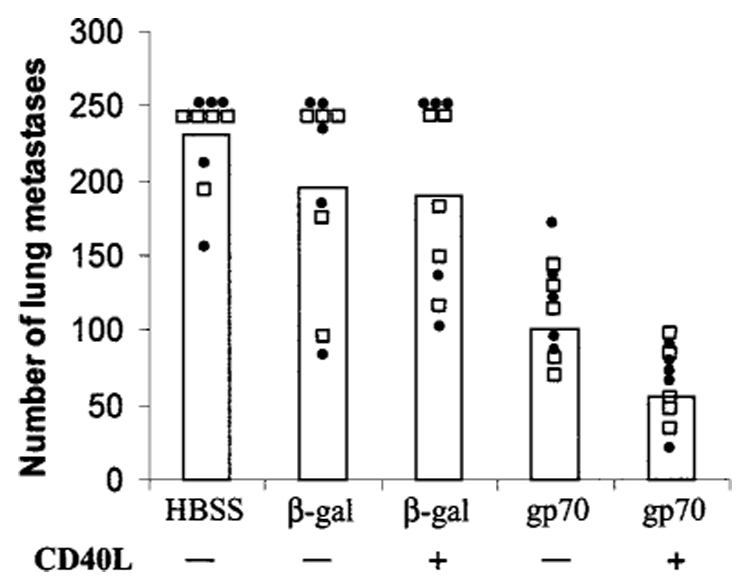
gp70-pulsed DCs inhibit established CT26 lung metastases. gp70 or β-gal peptide was pulsed onto DCs pretreated with CD40L-3T3 cells or control 3T3 cells. DCs were then administered to mice bearing 3-day lung metastases. Twelve days later lungs were removed and metastases enumerated. Mice receiving gp70-pulsed DCs had significantly fewer lung metastases than mice receiving HBSS or β-gal-pulsed DCs (P2 < 0.01). An additional significant reduction in the number of lung metastases was seen after treatment of DCs with CD40L before pulsing with gp70. Data from two experiments are presented here with experiment 1 represented by □ and experiment 2 by ●.
DCs Pulsed with p15E Peptide Can Treat Established Lung Metastases
To confirm the antitumor effectiveness of endogenous retroviral peptide-pulsed DCs, we next investigated the use of another retroviral antigen, p15E, in a different tumor model, B16 melanoma, in C57BL/6 mice. Mice were injected i.v. with 3 × 105 B16 melanoma cells to establish pulmonary metastases. Three days later mice received 4 × 105 peptide-pulsed DCs pretreated with CD40L. The effect of injection of IL-2 was also studied in this tumor model, because IL-2 has been demonstrated to augment antitumor effects in other studies (29). When lungs were examined 12 days later, it was clear that mice receiving p15E-pulsed DCs and IL-2 developed significantly fewer metastases than mice receiving HBSS or control β-gal-pulsed DCs (Fig. 2). The requirement for coadministration of IL-2 in this model was also evident, because mice receiving p15E-pulsed DCs without IL-2 developed similar numbers of lung metastases as control mice.
Fig. 2.
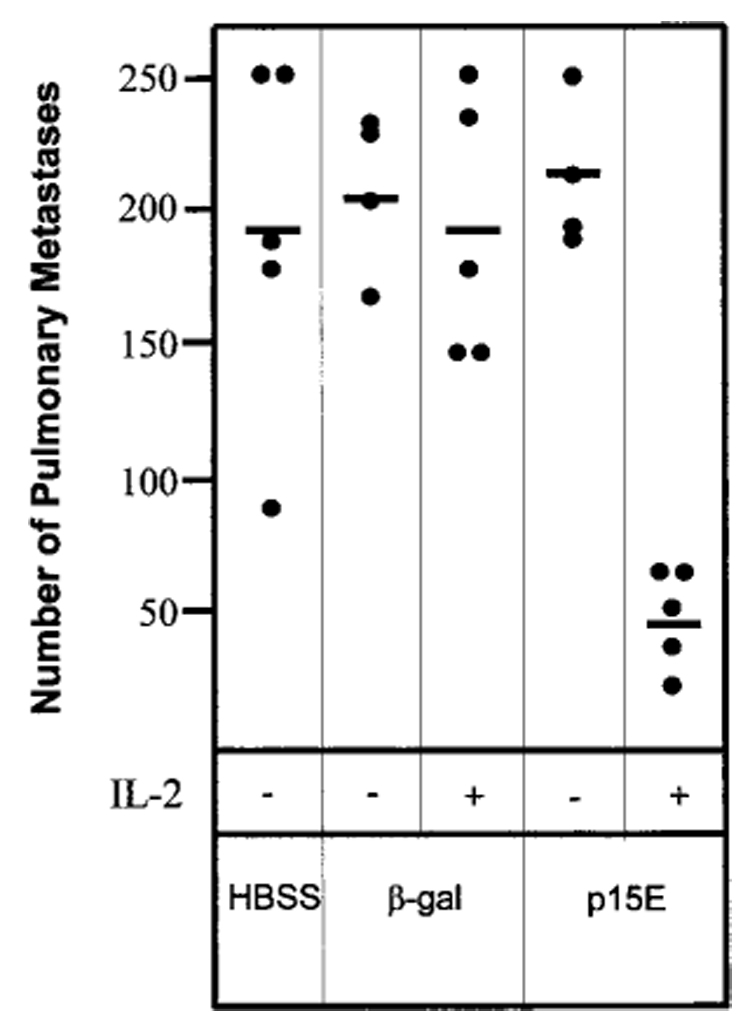
p15E peptide-pulsed DCs inhibit established B16 melanoma lung metastases. Mice bearing 3-day B16 lung metastases received CD40L-treated DCs pulsed with either β-gal or p15E peptide. Some groups of mice received IL-2 as described in “Materials and Methods.” Only mice receiving p15E peptide-pulsed DCs and IL-2 showed inhibition of lung metastases. Mice receiving β-gal peptide-pulsed DCs or p15E-pulsed DCs without IL-2 all developed similar numbers of lung metastases as control mice receiving HBSS only. This experiment was repeated with similar results.
DISCUSSION
In this study we investigated the use of endogenous retroviral antigens as immunogens in the CT26 colon carcinoma and B16 melanoma tumor models in mice. Previous studies demonstrated an antitumor response in BALB/c mice after immunization with CT26 modified to secrete granulocyte macrophage colony-stimulating factor, and this response was found to be at least partially directed against a peptide epitope of gp70 (10), but attempts to immunize with this peptide alone were unsuccessful. More recently, immunization with a modified gp70 peptide has been demonstrated to protect mice against tumor challenge (15). In the MC38 and B16 tumor models CTL can also be generated against another endogenous retroviral antigen, p15E (16, 17). In this paper, we describe for the first time active immunization against endogenous retroviral antigens in a treatment setting.
Other endogenous retroviral elements described previously include mouse mammary tumor virus-related sequences with ≤14 copies present in the genome. Mouse mammary tumor virus-related proteins can be detected in many tissues, but expression is highest in organs with exocrine function including mammary tumors and normal mammary gland (30). Human endogenous retroviral transcripts have been found in some human cancer cell lines (5–7), and recently an endogenous retroviral antigen recognized by T cells has been identified in some human melanomas. The association of endogenous retrovirus protein expression with some malignancies may make these viral proteins a potential target for cancer vaccines.
Recombinant vaccines based on pox viruses have been demonstrated to inhibit tumor growth in mice (18, 31). In addition antitumor effects have been demonstrated in mice using adenoviral or other viral vectors (32). Indeed, we found in this study that recombinant vaccinia was effective in immunizing against CT26 tumor. However, in our initial melanoma clinical trials using recombinant vaccinia and adenoviral vectors for immunization, we have found that preexisting neutralizing antibody may limit the effectiveness of these strategies (33). The use of recombinant fowlpox viruses may be effective at addressing this problem, because most patients do not have a preexisting neutralizing antibody against fowlpox.
Other means of initiating an immune response include the use of peptides derived from TAAs, which has been demonstrated to induce protective immunity in mice (34) and increase tumor-reactive T-cell precursors in melanoma patients (35). However, in some circumstances, administration of tumor-associated peptide has been demonstrated to induce tolerance and results in no protection against a tumor (36, 37). In general, in mice, vaccination with TAA peptide alone does not invoke a strong antitumor response.
Effective immunization is dependent on sufficient antigen-gaining access to significant numbers of APCs. DCs are effective APCs that express high levels of MHC and costimulatory molecules (38). One means of providing sufficient numbers of antigen-bearing APCs is by ex vivo expansion of DCs to large numbers (39) followed by pulsing with tumor peptide and delivery to the host. Tumor growth inhibition has been observed in mice receiving DCs pulsed with tumor antigen peptide (40) or transduced with tumor antigen genes (27). Our initial studies determined that DCs pulsed with gp70 endogenous retroviral peptide had a significant effect on 3-day CT26 lung metastases, although tumor inhibition was not complete. Activation of DCs with CD40L followed by pulsing with gp70 augmented the antitumor effect above that seen with gp70-pulsed DCs alone. CD40 is a Mr 50,000 dalton protein expressed on a variety of cells including B cells, monocytes, and DCs. Ligation of CD40 results in activation of DCs as demonstrated by increases in expression of costimulatory molecules (28). It is likely that the activation of DC by CD40L in this investigation resulted in increased stimulation of endogenous peptide-specific T cells leading to increased antitumor effects. Although we demonstrated a correlation between the generation of peptide-reactive T cells and increased antitumor immunity in the vaccinia studies described here, we did not determine this in the DC studies. It would be interesting to investigate this correlation in future studies involving immunization with peptide-pulsed DCs. In light of recent findings concerning the use of modified peptide epitopes in the generation of antitumor immunity (15) it would be interesting to determine whether peptide modification could additionally enhance the antitumor effects seen in our system.
The ability to immunize against endogenous retroviral antigens was confirmed in a second tumor model in another strain of mice (B16 melanoma in C57BL/6). In this part of the study CD40L-activated DCs pulsed with a peptide of p15E-produced tumor inhibition but only when mice also received IL-2. The reason for the requirement of IL-2 in the treatment is not clear, although it is likely that it was necessary for the optimal generation and activation of tumor-reactive T cells. Interestingly, IL-2 administration was not necessary for effective immunization of mice in the gp70/CT26 system described here. This may be attributable to inherent differences in the immunogenicity of the gp70 antigen in BALB/c mice compared with the p15E antigen in C57BL/6 mice. It is possible that the antitumor effect of immunization may be enhanced in the gp70/CT26 system with the addition of IL-2.
In summary, in this study we have demonstrated the ability to immunize against endogenous retroviral antigens. The use of recombinant vaccinia containing an immunodominant epitope of gp70 could significantly protect mice from subsequent tumor challenge, although no treatment of established lung metastases was observed. However, a significant treatment effect of established lung metastases was observed when either gp70 or p15E peptide was pulsed onto in vitro-generated, bone marrow-derived DCs, which were used to treat mice. The activation/differentiation state of DCs seems to be important in the generation of antitumor activity, because an additional increase in therapeutic effect was demonstrated after earlier in vitro treatment of DCs with CD40L. Because endogenous retroviral sequences may be antigenic in human tumors, this may have significance in the development of cancer vaccines.
ACKNOWLEDGMENTS
We thank Deborah R. Surman for help in virus preparation.
Footnotes
Supported by a National Health and Medical Research Council of Australia C. J. Martin Postdoctoral Research Fellowship (to M. H. K.).
The abbreviations used are: TAA, tumor-associated antigen; HERV, human endogenous retrovirus; DC, dendritic cell; IL, interleukin; β-gal, β-galactosidase; MuLV, murine leukemia virus; APC, antigen-presenting cell.
P. G. Coulie, personal communication.
Unpublished observations.
REFERENCES
- 1.Sauter M, Schommer S, Kremmer E, Remberger K, Dolken G, Lemm I, Buck M, Best B, Neumann-Haefelin D, Mueller-Lantzsch N. Human endogenous retrovirus K10: expression of Gag protein and detection of antibodies in patients with seminomas. J. Virol. 1995;69:414–421. doi: 10.1128/jvi.69.1.414-421.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wahlstrom T, Suni J, Nieminen P, Narvanen A, Lehtonen T, Vaheri A. Renal cell adenocarcinoma and retrovirus p30-related antigen excreted to urine. Lab. Investig. 1985;53:464–469. [PubMed] [Google Scholar]
- 3.Moyret CF, Bernard DJ, Maurizis JC, Chollet P, Plagne R. Detection of reverse transcriptase activity in human breast tumors. Anticancer Res. 1988;8:1279–1283. [PubMed] [Google Scholar]
- 4.Moshier JA, Luk GD, Huang RC. mRNA from human colon tumor and mucosa related to the pol gene of an endogenous A-type retrovirus. Biochem. Biophys. Res. Commun. 1986;139:1071–1077. doi: 10.1016/s0006-291x(86)80286-8. [DOI] [PubMed] [Google Scholar]
- 5.Andersson AC, Svensson AC, Rolny C, Andersson G, Larsson E. Expression of human endogenous retrovirus ERV3 (HERV-R) mRNA in normal and neoplastic tissues. Int. J. Oncol. 1998;12:309–313. doi: 10.3892/ijo.12.2.309. [DOI] [PubMed] [Google Scholar]
- 6.Lindeskog M, Blomberg J. Spliced human endogenous retroviral HERV-H env transcripts in T-cell leukaemia cell lines and normal leukocytes: alternative splicing pattern of HERV-H transcripts (Published erratum appears in J. Gen. Virol., 79: 212, 1998) J. Gen. Virol. 1997;78:2575–2585. doi: 10.1099/0022-1317-78-10-2575. [DOI] [PubMed] [Google Scholar]
- 7.Medstrand P, Blomberg J. Characterization of novel reverse transcriptase encoding human endogenous retroviral sequences similar to type A and type B retroviruses: differential transcription in normal human tissues. J. Virol. 1993;67:6778–6787. doi: 10.1128/jvi.67.11.6778-6787.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boller K, Janssen O, Schuldes H, Tonjes RR, Kurth R. Characterization of the antibody response specific for the human endogenous retrovirus HTDV/HERV-K. J. Virol. 1997;71:4581–4588. doi: 10.1128/jvi.71.6.4581-4588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boeke JD, Corces VG. Transcription and reverse transcription of retrotransposons. Annu. Rev. Microbiol. 1989;43:403–434. doi: 10.1146/annurev.mi.43.100189.002155. [DOI] [PubMed] [Google Scholar]
- 10.Huang AY, Gulden PH, Woods AS, Thomas MC, Tong CD, Wang W, Engelhard VH, Pasternack G, Cotter R, Hunt D, Pardoll DM, Jaffee EM. The immunodominant major histocompatibility complex class I-restricted antigen of a murine colon tumor derives from an endogenous retroviral gene product. Proc. Natl. Acad. Sci. USA. 1996;93:9730–9735. doi: 10.1073/pnas.93.18.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi H, Matsubara H, Yokota T, Kuwabara I, Kanno M, Koseki H, Isono K, Asano T, Taniguchi M. Molecular cloning and characterization of the gene encoding mouse melanoma antigen by cDNA library transfection. J. Immunol. 1992;149:1223–1229. [PubMed] [Google Scholar]
- 12.Yang JC, Perry-Lalley D. The envelope protein of an endogenous murine retrovirus is a tumor- associated T-cell antigen for multiple murine tumors. J. Immunother. 2000;23:177–183. doi: 10.1097/00002371-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Miyazawa M, Fujisawa R. Restriction of Friend virus-induced erythroid cell proliferation by CD4+ T-lymphocytes that recognize a single gp70 epitope. Leukemia. 1997;11 Suppl. 3:227–229. [PubMed] [Google Scholar]
- 14.Golgher D, Korangy F, Gao B, Gorski K, Jaffee E, Edidin M, Pardoll DM, Elliott T. An immunodominant MHC class II-restricted tumor antigen is conformation dependent and binds to the endoplasmic reticulum chaperone, calreticulin. J. Immunol. 2001;167:147–155. doi: 10.4049/jimmunol.167.1.147. [DOI] [PubMed] [Google Scholar]
- 15.Slansky JE, Rattis FM, Boyd LF, Fahmy T, Jaffee EM, Schneck JP, Margulies DH, Pardoll DM. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 2000;13:529–538. doi: 10.1016/s1074-7613(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 16.White HD, Roeder DA, Green WR. An immunodominant Kb-restricted peptide from the p15E transmembrane protein of endogenous ecotropic murine leukemia virus (MuLV) AKR623 that restores susceptibility of a tumor line to anti-AKR/Gross MuLV cytotoxic T lymphocytes (Published erratum appears in J. Virol., 68: 3452, 1994) J. Virol. 1994;68:897–904. doi: 10.1128/jvi.68.2.897-904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeh HJ, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J. Immunol. 1999;162:989–994. [PubMed] [Google Scholar]
- 18.Pardoll DM. Cancer vaccines. Nat. Med. 1998;4:525–531. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 19.Roy M, Waldschmidt T, Aruffo A, Ledbetter JA, Noelle RJ. The regulation of the expression of gp39, the CD40 ligand, on normal and cloned CD4+ T cells. J. Immunol. 1993;151:2497–2510. [PubMed] [Google Scholar]
- 20.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J. Exp. Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, Bronte V, Chen PW, Gritz L, Panicali D, Rosenberg SA, Restifo NP. Active immunotherapy of cancer with a nonreplicating recombinant fowlpox virus encoding a model tumor-associated antigen. J. Immunol. 1995;154:4685–4692. [PMC free article] [PubMed] [Google Scholar]
- 22.Chuah MK, Vandendriessche T, Morgan RA. Development and analysis of retroviral vectors expressing human factor VIII as a potential gene therapy for hemophilia A. Hum. Gene Ther. 1995;6:1363–1377. doi: 10.1089/hum.1995.6.11-1363. [DOI] [PubMed] [Google Scholar]
- 23.Daly T, Royal RE, Kershaw MH, Treisman J, Wang G, Li W, Herlyn D, Eshhar Z, Hwu P. Recognition of human colon cancer by T cells transduced with a chimeric receptor gene. Cancer Gene Ther. 2000;7:284–291. doi: 10.1038/sj.cgt.7700121. [DOI] [PubMed] [Google Scholar]
- 24.Gavin MA, Gilbert MJ, Riddell SR, Greenberg PD, Bevan MJ. Alkali hydrolysis of recombinant proteins allows for the rapid identification of class I MHC-restricted CTL epitopes. J. Immunol. 1993;151:3971–3980. [PubMed] [Google Scholar]
- 25.Bronte V, Carroll MW, Goletz TJ, Wang M, Overwijk WW, Marincola F, Rosenberg SA, Moss B, Restifo NP. Antigen expression by dendritic cells correlates with the therapeutic effectiveness of a model recombinant poxvirus tumor vaccine. Proc. Natl. Acad. Sci. USA. 1997;94:3183–3188. doi: 10.1073/pnas.94.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson K, Cresswell P, Gammon M, Hermes J, Williamson A, Zweerink H. Endogenously synthesized peptide with an endoplasmic reticulum signal sequence sensitizes antigen processing mutant cells to class I-restricted cell-mediated lysis. J. Exp. Med. 1991;174:489–492. doi: 10.1084/jem.174.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Specht JM, Wang G, Do MT, Lam JS, Royal RE, Reeves ME, Rosenberg SA, Hwu P. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J. Exp. Med. 1997;186:1213–1221. doi: 10.1084/jem.186.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 cross-linking. J. Exp. Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karp SE, Farber A, Salo JC, Hwu P, Jaffe G, Asher AL, Shiloni E, Restifo NP, Mule JJ, Rosenberg SA. Cytokine secretion by genetically modified nonimmunogenic murine fibrosarcoma. Tumor inhibition by IL-2 but not tumor necrosis factor. J. Immunol. 1993;150:896–908. [PMC free article] [PubMed] [Google Scholar]
- 30.Acha-Orbea H, MacDonald HR. Superantigens of mouse mammary tumor virus. Annu. Rev. Immunol. 1995;13:459–486. doi: 10.1146/annurev.iy.13.040195.002331. [DOI] [PubMed] [Google Scholar]
- 31.Bernards R, Destree A, McKenzie S, Gordon E, Weinberg RA, Panicali D. Effective tumor immunotherapy directed against an oncogene-encoded product using a vaccinia virus vector. Proc. Natl. Acad. Sci. USA. 1987;84:6854–6858. doi: 10.1073/pnas.84.19.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen PW, Wang M, Bronte V, Zhai Y, Rosenberg SA, Restifo NP. Therapeutic antitumor response after immunization with a recombinant adenovirus encoding a model tumor-associated antigen. J. Immunol. 1996;156:224–231. [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenberg SA, Zhai Y, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Seipp CA, Einhorn JH, Roberts B, White DE. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J. Natl. Cancer Inst. 1998;90:1894–1900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW, ter Schegget J, Melief CJ, Kast WM. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur. J. Immunol. 1993;23:2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Dudley ME, Schwarz SL, Spiess PJ, Wunderlich JR, Parkhurst MR, Kawakami Y, Seipp CA, Einhorn JH, White DE. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma [see comments] Nat. Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aichele P, Brduscha-Riem K, Zinkernagel RM, Hengartner H, Pircher H. T cell priming versus T cell tolerance induced by synthetic peptides. J. Exp. Med. 1995;182:261–266. doi: 10.1084/jem.182.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toes RE, Blom RJ, Offringa R, Kast WM, Melief CJ. Enhanced tumor outgrowth after peptide vaccination. Functional deletion of tumor-specific CTL induced by peptide vaccination can lead to the inability to reject tumors. J. Immunol. 1996;156:3911–3918. [PubMed] [Google Scholar]
- 38.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 39.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJ, Ildstad ST, Kast WM, Deleo AB. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat. Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]


