Abstract
Oligoanions such as sodium triphosphate or GTP prevent and/or reverse vinblastine-induced polymerization of tubulin. We now show that the anions of glutamate-rich extreme C termini of tubulin are similarly involved in the regulation of the vinblastine effect. Cleavage of the C termini by limited proteolysis with subtilisin enhances vinblastine-induced tubulin polymerization and abolishes the anion effect. Only the β-tubulin C terminus needs to be removed to achieve these changes and the later cleavage of the α-tubulin C terminus has little additional effect. In fact, vinblastine concentrations >20 μM block cleavage of the α-tubulin C terminus in the polymer, whereas cleavage of the β-tubulin C terminus proceeds unimpeded over the time used. The vinblastine effect on tubulin polymerization is also highly pH-dependent between pH 6.5 and 7.5; this is less marked, but not absent, after subtilisin treatment. A working model is proposed wherein an anionic domain proximal to the extreme C terminus must interact with a cationic domain to permit vinblastine to promote polymerization. Both exogenous and extreme C-terminal anions compete for the cationic domain with the proximal anionic domain to prevent vinblastine-induced polymerization. We conclude that the electrostatic regulation of tubulin polymerization induced by vinblastine resides primarily in the β-tubulin C terminus but that additional regulation proximal in the tubulin molecule also plays a role.
Tubulin is the main structural component of microtubules and is the target of antitumor drugs such as vinblastine, colchicine, and Taxol, which interfere with microtubule function. Vinblastine affects tubulin differently at different concentrations: at substoichiometric concentrations (<1 μM), it diminishes microtubule dynamics (1); at intermediate concentrations (<10 μM), it inhibits the formation of microtubules (2); and at >10 μM, it promotes polymerization or aggregation into spirals and other polymers (3–5). In previous studies (6), we showed that vinblastine-induced tubulin polymerization proceeds in two steps, the formation of smaller oligomers followed by polymerization to larger structures. Oligoanions such as GTP, sodium triphosphate or suramine inhibit the formation of the bigger polymers (6). The region in the protein where these oligoanions interact is not known. We concluded that vinblastine-induced polymerization was electrostatically regulated.
Both the α and β monomers of tubulin are acidic showing a high surface charge density. The C termini of both monomers have the highest charge density and can be cleaved with subtilisin at the extreme C-terminal portions (7, 8). Subtilisin cleaves α-tubulin between Asp-438 and Ser-439 and β-tubulin between Gln-433 and Gly-434 (9). The cleaved protein is referred to as tubulin S or αsβs, and because of the more rapid cleavage of β tubulin, its formation proceeds through an intermediate called αβs. Both the α-tubulin and β-tubulin C termini are rich in negatively charged amino acid residues and these have been shown to play a major role in microtubule assembly on the basis of charge repulsion. To determine whether or not these C termini can influence the effects of vinblastine, limited proteolysis with subtilisin has been carried out. We report herein that the extent and rate of vinblastine-induced tubulin polymerization is greater in tubulin S than in tubulin and that the oligoanion inhibition of this process in tubulin is completely abolished by removal of the C termini, particularly the β-tubulin C terminus.
MATERIALS AND METHODS
Reagents.
GTP, vinblastine sulfate, sodium triphosphate, and subtilisin (BPN) were from Sigma; suramine was from Imperial Chemical Industries. Vinblastine-4′-anthranilate was prepared as described (10). Rat brain tubulin was prepared by polymerization–depolymerization cycles as described (11, 12) and was >99% pure in overloaded SDS/10% polyacrylamide gels supported on GelBond film (FMC Bioproducts). Tubulin S was prepared as described (8, 13).
Polymerization.
Polymerization was carried out at 25°C in Mes assembly buffer (0.1 M Mes adjusted to pH 6.9 with NaOH/1 mM EGTA/1 mM MgCl2) containing 10% dimethyl sulfoxide. Polymerization was measured as turbidity at 400 nm at 25°C in a Cary 219 spectrophotometer in 3-mm light-path-masked cuvettes and a band width of 1 nm. Measurements were started 20 sec after addition of tubulin. The formation of smaller polymers was measured by 90° light scattering in a Perkin–Elmer MPF 66 fluorimeter operating in the uncorrected mode with low gain, at 400 nm, 5-nm slits and masked 3-mm light-path cuvettes. Polymer mass was determined at steady state (30 min at 25°C) by centrifugation in an Airfuge at room temperature and 148,000 × gmax for 5 min. Pellet protein was measured by the bicinchoninic acid method with BSA as standard according to the manufacturers directions (Pierce). With lower (20 μM) vinblastine concentrations, pellets were collected under mineral oil in a Ti70.1 rotor for 20 min at 400,000 × g and 25°C and processed as above.
Proteolysis.
Tubulin and vinblastine-induced polymers were subjected to limited 1:100 (wt/wt) subtilisin (BPN) proteolysis at 25°C as described (13). Aliquots were removed at different times and proteolysis was stopped with 1% phenylmethylsulfonyl fluoride. Samples were immediately boiled in loading solution and analyzed on SDS/10% polyacrylamide gels. Gels were scanned and quantitated with diversity 1 software.
RESULTS
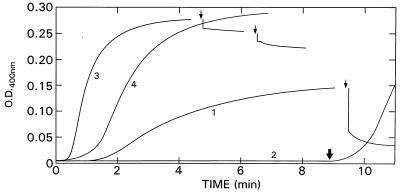
To study the role of the C termini of tubulin in vinblastine-induced polymerization, samples were treated with subtilisin, which cleaves α- and β-tubulin after residues Asp-438 and Gln-433, respectively (9). Curve 1 of Fig. 1 shows control vinblastine-induced polymerization in the absence of GTP, and curve 2 shows the complete inhibition of this process by 0.5 mM GTP. When these reactions were carried out in the presence of subtilisin (1:100), there was a large increase in the rate and smaller increase in the extent of turbidity generation, as well as a reduction in the latent period (curve 3). The increase in turbidity in curve 3 was greater than that obtained when microtubule assembly was promoted by subtilisin treatment, i.e., in the absence of vinblastine (data not shown). However, when the subtilisin effect was assessed as changes in pelletable protein, the excess pelletable protein was roughly the same without (microtubules) and with (spirals) vinblastine. The difference in OD is thus most likely due to a difference in the scattering coefficient of the two polymers. We also can deduce that the changes in critical concentration for the two polymerization processes produced by subtilisin must have been nearly the same. Experiments with tubulin S, in which the C termini had been removed before addition of vinblastine, yielded identical results (data not shown). Remarkably, subtilisin abolished the inhibition of vinblastine-induced polymerization caused by GTP, although the rate was slower and the reduction of the latent period was less (curve 4); however, the extent of turbidity generation was the same as for curve 3. Addition of subtilisin to the GTP-inhibited sample after 9 min rapidly relieved the inhibition (thick arrow on curve 2). Conversely, addition of GTP or sodium triphosphate to preformed polymers led to a sharp decrease in turbidity (curve 1, arrow); however, after subtilisin treatment, preformed polymers were no longer sensitive to GTP (curve 3, left arrow) or sodium triphosphate (curve 3, right arrow). The small decreases in OD are dilution effects. These results clearly show that the C termini of tubulin exert two major effects on the response to vinblastine: a change in the apparent critical concentration for polymerization and abolition of oligoanion reversal of the vinblastine effect.
Figure 1.
Effect of subtilisin on vinblastine-induced tubulin polymerization. Rat brain tubulin (2 mg/ml) was polymerized at 25°C, with 10% dimethyl sulfoxide and 50 μM vinblastine. Curves: 1, control, 1 mM GTP added at arrow; 2, with 0.5 mM GTP, subtilisin (1:100) added at thick arrow; 3, subtilisin added at beginning, left arrow indicates 1 mM GTP was added, and right arrow indicates 1 mM sodium triphosphate was added; 4, with 0.5 mM GTP and subtilisin added at beginning.
As shown above, changes in turbidity do not necessarily reflect changes in polymer mass; therefore, in similar experiments to the above, we followed the amount of protein that could be pelleted in an Airfuge (148,000 × gmax 5 min at room temperature). Of a total of 126 μg of tubulin, 102 ± 3 μg were pelleted in controls after a 30-min incubation at 25°C with 50 μM vinblastine. In the presence of 0.5 mM GTP (or sodium triphosphate), the solution, which was now no longer turbid, still contained 21 ± 1 μg of pelletable tubulin. Treatment with 1:100 subtilisin, which yielded a large increase in turbidity (Fig. 1, curves 3 and 4) increased pelletable tubulin to 120 ± 5 μg; this was not changed in the presence of 0.5 mM GTP (123 ± 3 μg of tubulin). Similar results were obtained when subtilisin treatment preceded vinblastine addition by 30 min. Note that we have shown previously (6) that the GTP effect is not due to Mg2+ depletion.
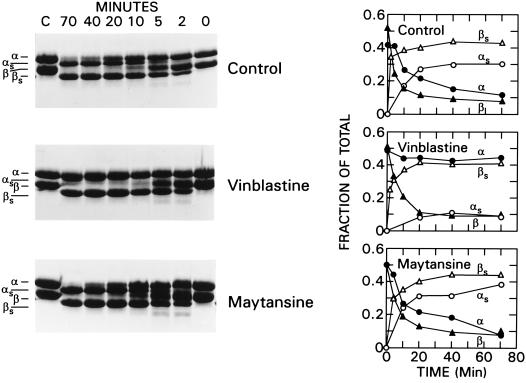
The subtilisin effect is very rapid and plateau turbidity is attained in <10 min (Fig. 1, curves 3 and 4). Because it is known that subtilisin cleaves β-tubulin preferentially over α-tubulin (13), we compared the observed time above to the rates of cleavage of β- and α-tubulin (Fig. 2 Top). Under the present incubation conditions, there was substantial hydrolysis of β-tubuilin by 2 min and complete removal of the β-tubulin C terminus by 20 min, yielding the hybrid dimer αβs as the major product. Cleavage of the α-tubulin C terminus, to yield tubulin S (αsβs), was much slower, remaining incomplete even at 70 min. Quantitation by scanning of this gel is depicted in Fig. 2 Top Right. Because the vinblastine effect on turbidity and the oligoanion effects are both rapid, their time course coincides best with the early cleavage of β-tubulin (at residue 433) and the formation of αβs, which has a lower critical concentration than native tubulin (8). At the same time, it is apparent that not all of the β-tubulin C termini need to be cleaved to enhance the vinblastine effect and diminish the oligoanion effect. This is reminiscent of the finding that only a small mole fraction of tubulin S will nucleate enough native tubulin to achieve a substantial reduction in the critical concentration for microtubule assembly (8). Thus, the indirect effects of reduction in charge must also be considered in the interpretation of the subtilisin-induced cleavage.
Figure 2.
Limited subtilisin digestion of tubulin, vinblastine-treated tubulin, or maytansine-treated tubulin as a function of time. Tubulin (2.0 mg/ml) in Mes assembly buffer containing 10% dimethyl sulfoxide was exposed to 50 μM vinblastine or 50 μM maytansine for 25 min at 25°C followed by addition of 1:100 subtilisin. Digestion was stopped with 1% phenylmethylsulfonyl fluoride, and samples were electrophoresed on SDS/10% gels. (Left) Coomassie stain. (Right) Fraction of tubulin as native or digested monomers. (Top) Control without drugs. (Middle) Pretreatment with vinblastine. (Bottom) Pretreatment with maytansine. •, ▴, ○, and ▵ refer to α-, β-, αs-, and βs-tubulins, respectively.
An unexpected finding was the protection promoted by vinblastine against subtilisin proteolysis at residue α438. In Fig. 2 Center, 50 μM vinblastine was present during the digestion. The β-tubulin C terminus was cleaved at essentially the same rate as in the controls, whereas the α-tubulin C terminus was hardly cleaved even by 70 min, yielding only ≈10% αs. The protection of the α-tubulin C terminus might be due either to occupancy of the Vinca binding site or to the inaccessibility of the cleavage site α438 in the polymer. The former possibility was investigated with 50 μM maytansine, which binds at the Vinca site but does not polymerize tubulin. Fig. 2 Bottom shows that the presence of maytansine has little effect on the rates of hydrolysis of either β- or α-tubulin C termini and quantitation is like that of the controls. It is possible that the differences between maytansine and vinblastine are due to occupancy of different portions of the binding site on β-tubulin (residues 175–213) (10). This sequence contains helix H5, β-strand B6, and helix H6 (14), which form a pocket near the exchangeable GTP site. However, this locus is not near the β-tubulin C terminus, and from current data (14), it is also not near the α-tubulin C terminus of the adjacent dimer. Thus, direct blockage of the cleavage site by vinblastine seems improbable, and these findings suggest that the protection of this α cleavage site is likely to be the result of the polymerized state in which it appears to be buried more extensively than it is in the absence of vinblastine.
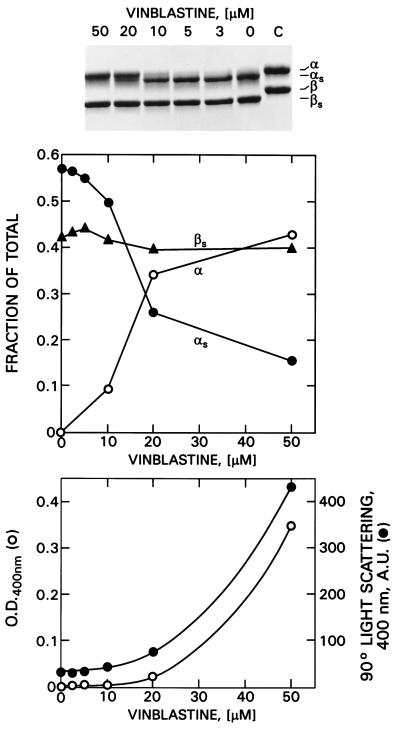
The protection of α-tubulin against subtilisin proteolysis is a function of the vinblastine concentrations as shown in Fig. 3 Top and Center. At low concentrations of vinblastine, the α-tubulin C termini are readily cleaved, whereas at ≥20 μM vinblastine, the cleavage site is protected. To determine whether or not sufficient polymerization occurs at this vinblastine concentration to account for the protection, we measured the fraction of total tubulin that could be pelleted in an Airfuge. When tubulin was incubated with 20 μM vinblastine and subsequently centrifuged at room temperature for 10 min at 148,000 × gmax, only 30–35% of the protein was pelletable even though there was nearly complete protection of the α-tubulin C terminus. To consider the possibility that a smaller polymer might account for the protection, we measured both turbidity and 90° light scattering as a function of vinblastine concentration (Fig. 3 Bottom). It is apparent that although little turbidity occurs at 20 μM vinblastine, substantial 90° light scattering is observed, suggesting the presence of a smaller polymer that might require greater g forces to sediment. Accordingly, we centrifuged the above mixture at 25°C for 20 min at 400,000 × g in a Ti70.1 rotor. As control, we used an equivalent concentration of maytansine. With 20 μM vinblastine, 85% of the total tubulin was pelleted, whereas only 8% of the maytansine–tubulin complex was pelleted. Thus, the formation of smaller polymers at this concentration of vinblastine appears to be sufficient to make the α438–439 bond less accessible to subtilisin.
Figure 3.
Dependence of subtilisin digestion on vinblastine concentration. Tubulin (2 mg/ml) was preincubated with different concentrations of vinblastine at 25°C for 25 min followed by subtilisin (1:100) digestion for 50 min. After addition of 1% phenylmethylsulfonyl fluoride, samples were electrophoresed on SDS/10% gels. (Top) Coomassie stain. (Middle) Fraction of tubulin as native or digested monomers. (Bottom) Comparison of turbidity (○) and 90° light scattering (•) under the same conditions.
If inhibition of vinblastine-induced polymerization of tubulin requires the additive effect of the β-tubulin C-terminal carboxylates and the extrinsic oligoanions, as suggested by the above experiments, then larger concentrations of GTP or sodium triphosphate might be expected to compensate for the loss of the carboxylates in tubulin S. Demonstration of this was partially successful but it was necessary to reduce the vinblastine concentration to 10 μM and increase the oligoanion concentration to 10 mM. Under these conditions, GTP increased the latent period and decreased the rate of polymerization, whereas at the same concentration of sodium triphosphate polymerization was nearly totally suppressed (data not shown).
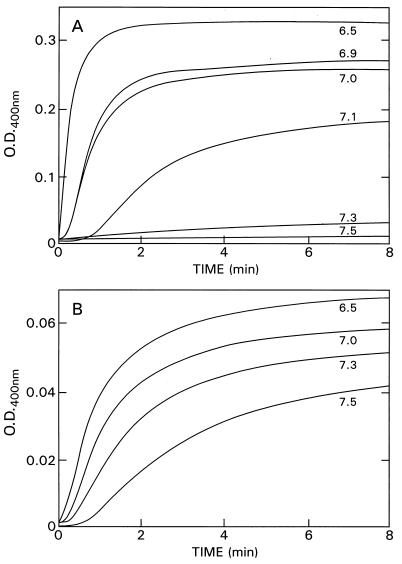
It remained to be determined whether or not regulation of vinblastine-induced polymerization occurs exclusively at the β-tubulin C terminus or also occurred elsewhere in the tubulin molecule. Because the C-terminal carboxylates are not expected to titrate at pH 6.9 (e.g., ref. 15), it seemed possible that titration of the vinblastine effect from pH 6.5 to 7.5 might reveal an additional regulating domain. Therefore, we compared the pH dependence of the vinblastine-induced polymerization of tubulin and tubulin S. With native tubulin, vinblastine-induced polymerization shows a remarkable decrease in the rate and extent of turbidity generation as the pH is increased from pH 6.5 to 7.3, and the lag phase increased pari passu (Fig. 4A). At pH 7.3 virtually no turbidity can be monitored at 400 nm. This is not an effect of the changes in ionic strength over this pH range because the ionic strength varied only slightly between pH 6.5 and 7.5 (NaCl equivalent was from 38 to 54 mM NaCl), and addition of NaCl to the pH 6.5 sample to achieve ionic strength equal to the pH 7.5 sample did not alter the results. It was, however, possible that the changes seen with pH signaled the formation of a different polymer. Because it is difficult to obtain electron micrographs not disturbed by changes during fixation, we used wavelength dependence of light scattering to detect different polymers. Between pH 6.5 and 7.2, no change in this relationship occurred. With prolonged incubation at pH 7.3 however, there was a sudden change in the scattering profile as a function of wavelength between 500 and 335 nm. The nature of this sudden change is not known but is under investigation. With tubulin S, vinblastine-induced polymerization shows markedly less striking pH dependence, but decreased polymerization still occurs at higher pH values (Fig. 4B). Thus, a titratable domain proximal to the C termini is also likely to be involved in the regulation of the vinblastine effect, although the β-tubulin C terminus is the primary regulator. It may, in turn, influence regulation by the secondary site.
Figure 4.
pH dependence of vinblastine-induced polymerization of tubulin and tubulin S. (A) Tubulin (1.4 mg/ml) with 50 μM vinblastine at 25°C. (B) Tubulin S (0.15 mg/ml) with 10 μM vinblastine at 25°C.
DISCUSSION
Vinblastine-induced tubulin polymerization proceeds through at least two steps: formation of a small polymer followed by formation of a larger polymer, the familiar spirals or spiral aggregate. Oligoanions such as GTP, sodium triphosphate, or suramine nonspecifically inhibit formation of the larger polymer, suggesting that the vinblastine-induced polymerization is electrostatically regulated (6). Because microtubule assembly is also electrostatically regulated, in large part by anionic repulsion at the C termini (16), we used limited subtilisin proteolysis (producing tubulin S) to assess the role of these C termini in vinblastine-induced polymer formation and its oligoanion-mediated reversal. Cleavage of the C termini increases the rate, and to a much lesser degree the extent, of turbidity generation by vinblastine compared with native tubulin. This also has been shown earlier (17, 18). The limited change in polymer mass clearly suggests the formation of a different polymer. The surprising finding in the present study is that subtilisin cleavage can completely abolish the oligoanion-mediated inhibition of the vinblastine effect. These two results of subtilisin cleavage occur on a time scale commensurate with the cleavage of β-tubulin, not α-tubulin. The subsequent cleavage of α-tubulin has little additional effect on the response to vinblastine or oligoanions. In fact, vinblastine significantly hinders cleavage at α438 by subtilisin. By contrast, in microtubules, both α- and β-tubulin C termini are accessible to subtilisin (13). Moreover, inhibition of vinblastine-induced polymerization of tubulin appears to require the additive effects of the β-tubulin C-terminal carboxylates and the participation of another anion-sensitive site proximal to the C terminus. This is shown by the persistence, albeit less extensive, of the oligoanion effect after removal of the C termini and the partial restoration of anion inhibition in tubulin S by the use of high anion concentrations. The interaction of the C termini with vinblastine differs markedly from that with colchicine where the removal of the β-tubulin C terminus has little effect, whereas the absence of both C termini completely changes colchicine binding (19).
To reconcile these findings, we have constructed a crude working model that accounts for the effects of vinblastine, oligoanions, and the β-tubulin C-terminal carboxylates. In this scheme vinblastine effects on polymerization require a charge-based interaction between a positive domain (probably, but not necessarily, on β-tubulin) and a negatively charged domain proximal to the cleavage site for subtilisin at β433. The oligoanions are then seen as shielding the positive domain and destabilizing its interactions with the negatively charged domain. The Glu-rich distal β-tubulin C terminus would be seen as competing with the proximal negative domain for the positive domain, thus also causing destabilization, an effect that is relieved by β-tubulin cleavage with subtilisin. In a sense these two anionic effects are additive and the removal of the β-tubulin C terminus can be partially compensated by use of much higher oligoanion concentrations.
If this model has any validity, the presumptive positive domain might be sensitive to pH titration over the range tolerated by tubulin. Accordingly, we measured vinblastine-induced polymerization between pH 6.5 and 7.5. As shown in Fig. 4, the great sensitivity of the turbidity to rather small changes in pH is striking. In this pH range, carboxylates are not likely to be titrated (e.g., ref. 15), and because there are no other titratable amino acids, this suggests that the pH effect does not occur in the β-tubulin C terminus but possibly at a His residue proximal to the cleavage site in tubulin. The locus has not been identified but appears not to be the Vinca binding site because the binding-dependent emission of a fluorescent vinblastine analogue is not altered between pH 6.5 and 7.5. In any case, this titration is entirely consistent with the above model. Vinblastine causes no major structural changes in tubulin as measured by CD (20); nevertheless, a number of long-range effects of vinblastine has been described. Numerous investigators have found vinblastine-induced stabilization of the colchicine binding site, and the fluorescence of tubulin-bound 1-anilino-8-naphthalene sulfonate at another unrelated site shows increased quantum yields under the influence of vinblastine (21). Vinblastine binding also reverses a local unfolding around residue β390 caused by colchicine, as well as the colchicine-stimulated GTPase activity (22). Yet more proximal in the linear sequence of β-tubulin, the cross-linking of Cys-239 to Cys-354 by N,N′-ethylene bis(iodoacetamide) is enhanced by vinblastine (23).
The C termini of tubulin play a role in the dimer–polymer equilibrium (16), as attachment points for microtubule-associated proteins and cytoplasmic dynein (24–26), in the enhancement of the pH sensitivity of the vinblastine-induced polymerization process (see above), and as possible binding sites for Ca2+ (27). However, high-affinity binding can be demonstrated only at unphysiologically low ionic strength (28, 29). Although it has not always been clear to which C terminus these functions should be assigned, the present results and several earlier ones show a clear distinction between the α- and β-tubulin C termini:
(i) The β-tubulin C terminus is more accessible to subtilisin (refs. 8 and 16 and the present study).
(ii) Each C terminus individually promotes lowering of the critical concentration in an additive manner (8).
(iii) Each C terminus contributes to 4′,6-diamidino-2-phenylindole fluorescence with kinetics commensurate with β- and α-tubulin cleavage rates, respectively (30).
(iv) The α-tubulin C terminus is more important in determining colchicine binding properties (19) despite the fact that the binding site is located on β-tubulin (31, 32).
(v) Despite the high degree of identity between the C termini, the β-tubulin C terminus binds microtubule-associated protein 2 or tau, whereas the α-tubulin C terminus binds these proteins only weakly (25).
(vi) The β-tubulin C terminus enhances the polymerization response to vinblastine, whereas the α-tubulin C terminus does not, as shown above.
(vii) Removal of the β-tubulin C terminus abolishes the inhibitory effect of oligoanions on vinblastine-induced polymerization, whereas the α-tubulin C terminus contributes little additional effect, as shown above.
Thus, it is clear that the C termini can act independently and/or differently on the properties of tubulin both in the dimer and in polymers. We conclude that the C terminus of β-tubulin plays an important role in the formation of vinblastine-induced polymers, its regulation by oligoanions, and its sensitivity to small pH changes but that conversely, vinblastine influences the properties of the α- and β-tubulin C termini.
Acknowledgments
We thank Leslie Knipling for generous supplies of tubulin and tubulin S and Dr. Dan Sackett for helpful discussions.
References
- 1.Toso R J, Jordan M A, Farrel K W, Matsumoto B, Wilson L. Biochemistry. 1993;32:1285–1293. doi: 10.1021/bi00056a013. [DOI] [PubMed] [Google Scholar]
- 2.Wilson L, Jordan M A, Morse A, Margolis R L. J Mol Biol. 1982;159:125–149. doi: 10.1016/0022-2836(82)90035-3. [DOI] [PubMed] [Google Scholar]
- 3.Bensch K, Malawista S E. Nature (London) 1968;218:1176–1177. doi: 10.1038/2181176a0. [DOI] [PubMed] [Google Scholar]
- 4.Hamel E. Microtubule Proteins. Boca Raton, FL: CRC; 1990. pp. 89–191. [Google Scholar]
- 5.Himes R H. Pharmacol Ther. 1991;52:257–267. doi: 10.1016/0163-7258(91)90081-v. [DOI] [PubMed] [Google Scholar]
- 6.Rai S S, Wolff J. Eur J Biochem. 1997;250:425–431. doi: 10.1111/j.1432-1033.1997.0425a.x. [DOI] [PubMed] [Google Scholar]
- 7.Serrano L, Avila J, Maccioni R B. Biochemistry. 1984;23:4675–4681. doi: 10.1021/bi00315a024. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharyya B, Sackett D L, Wolff J. J Biol Chem. 1985;260:10208–10216. [PubMed] [Google Scholar]
- 9.Redeker V, Melki R, Prome D, LeCaer J P, Rosssier J. FEBS Lett. 1992;313:185–192. doi: 10.1016/0014-5793(92)81441-n. [DOI] [PubMed] [Google Scholar]
- 10.Rai S S, Wolff J. J Biol Chem. 1996;271:14707–14711. doi: 10.1074/jbc.271.25.14707. [DOI] [PubMed] [Google Scholar]
- 11.Hamel E, Lin C, M. Biochemistry. 1984;23:4172–4184. [Google Scholar]
- 12.Sackett D L, Knipling L, Wolff J. Protein Expression Purif. 1991;2:390–393. doi: 10.1016/1046-5928(91)90099-5. [DOI] [PubMed] [Google Scholar]
- 13.Sackett D L, Wolff J. J Biol Chem. 1986;261:9070–9076. [PubMed] [Google Scholar]
- 14.Nogales E, Wolf S G, Downing K H. Nature (London) 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 15.Kohn W D, Monero O D, Kay C M, Hodges R S. J Biol Chem. 1995;270:25495–25506. doi: 10.1074/jbc.270.43.25495. [DOI] [PubMed] [Google Scholar]
- 16.Sackett D L, Bhattacharyya B, Wolff J. J Biol Chem. 1985;260:43–45. [PubMed] [Google Scholar]
- 17.Serrano l, Valencia A, Caballero R, Avila J. J Biol Chem. 1986;261:7076–7081. [PubMed] [Google Scholar]
- 18.White E A, Burton P R, Himes R H. Cell Motil Cytoskeleton. 1987;7:31–38. doi: 10.1002/cm.970070105. [DOI] [PubMed] [Google Scholar]
- 19.Mukhopadhyay K, Parrack P K, Bhattacharyya B. Biochemistry. 1990;29:6845–6850. doi: 10.1021/bi00481a013. [DOI] [PubMed] [Google Scholar]
- 20.Lee J C, Harrison D, Timasheff S N. J Biol Chem. 1975;250:9276–9282. [PubMed] [Google Scholar]
- 21.Bhattacharyya B, Wolff J. Arch Biochem Biophys. 1975;167:264–269. doi: 10.1016/0003-9861(75)90462-2. [DOI] [PubMed] [Google Scholar]
- 22.Sackett D L. Biochemistry. 1995;34:7010–7019. doi: 10.1021/bi00021a012. [DOI] [PubMed] [Google Scholar]
- 23.Luduena R F, Roach M C. Pharmacol Ther. 1991;49:133–152. doi: 10.1016/0163-7258(91)90027-j. [DOI] [PubMed] [Google Scholar]
- 24.Serrano L, De La Torre J, Maccioni R B, Avila J. Proc Natl Acad Sci USA. 1984;81:5989–5993. doi: 10.1073/pnas.81.19.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Littauer U Z, Giveon D, Thierauf M, Ginsburg I, Ponstingl H. Proc Natl Acad Sci USA. 1986;83:7162–7166. doi: 10.1073/pnas.83.19.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paschal B M, Obar R A, Vallee R B. Nature (London) 1989;342:569–572. doi: 10.1038/342569a0. [DOI] [PubMed] [Google Scholar]
- 27.Solomon F. Biochemistry. 1977;16:358–363. doi: 10.1021/bi00622a003. [DOI] [PubMed] [Google Scholar]
- 28.Serrano L, DeLaTorre J, Luduena R D, Avila J. Arch Biochem Biophys. 1986;249:611–615. doi: 10.1016/0003-9861(86)90040-8. [DOI] [PubMed] [Google Scholar]
- 29.Wolff J. Colloque INSERM. 1988;171:477–490. [Google Scholar]
- 30.Ortiz M, Lagos R, Monasterio O. Arch Biochem Biophys. 1993;279:159–164. doi: 10.1006/abbi.1993.1267. [DOI] [PubMed] [Google Scholar]
- 31.Uppuluri S, Knipling L, Sackett D L, Wolff J. Proc Natl Acad Sci USA. 1993;90:11598–11602. doi: 10.1073/pnas.90.24.11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai R, Pei X-F, Boye O, Getahun Z, Gover S, Bekisz J, Nguyen N Y, Brossi A, Hamel E. J Biol Chem. 1996;271:12639–12645. doi: 10.1074/jbc.271.21.12639. [DOI] [PubMed] [Google Scholar]